脂基配方的新发展:通过近红外高光谱成像改善润湿性和均匀的API固体分散。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-06
DOI:10.1016/j.ejpb.2025.114864
引用次数: 0
摘要
脂基配方已经成功地应用于改善活性药物成分(api)的水溶性,然而,系统的有限润湿性是瓶颈。本研究以脂肪酸聚甘油酯(PGFA)为主要成分,六甘油酯(PG6)为润湿剂,开发了一种脂基体系。选择BCSⅱ类化合物非洛地平作为模型API。两种不同的温度(95 °C和55 °C)用于生产API负载的熔融铸造样品,含和不含PG6。采用近红外高光谱成像(NIR-HSI)作为评价和优化非洛地平在脂质体系中的分散度的先进工具。建立的NIR-HSI模型具有良好的线性度和准确性(R2Y = 80.999,RMSEC = 0.084),可作为筛选和优化原料药分散度的有力工具。采用95 °C制备熔铸样品,增强了非洛地平的分子分布,药物非晶化和均匀分散在基质内。非洛地平抑制脂质晶体生长。在所有脂基制剂中观察到浓度依赖性API释放(一级动力学)。原料药的释放速率受脂质晶体大小、体系的润湿性和原料药在脂基基质中的固体分散类型的影响。深入了解这些参数,并结合NIR-HSI等新颖而强大的分析工具的应用,为开发具有定制API释放的先进脂质配方铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
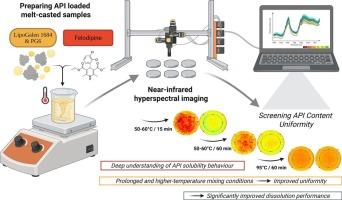
Novel development of lipid-based formulations: Improved wettability and homogeneous API solid dispersion visualised via near-infrared hyperspectral imaging
Lipid-based formulations have been successfully applied to improve the aqueous solubility of active pharmaceutical ingredients (APIs), however, with the bottleneck of limited wettability of the system.
In this study, a lipid-based system was developed using polyglycerol ester of fatty acids (PGFA) as the main component and hexaglycerol (PG6) as a wetting agent. Felodipine, a BCS class II compound was selected as a model API. Two different temperatures (95 °C and 55 °C) were used to produce API loaded melt-casted samples, with and without PG6. Near-infrared hyperspectral imaging (NIR-HSI) was used as an advanced tool to evaluate and optimize felodipine dispersion in the lipid-based system.
The established NIR-HSI model demonstrated excellent linearity and accuracy (R2Y = 80.999, RMSEC = 0.084) and acted as a powerful tool for screening and optimizing the API dispersibility. Applying 95 °C for producing melt-casted samples enhanced the molecular distribution of felodipine, drug amorphization and uniform dispersion within the matrix. Felodipine inhibited the crystal growth of the lipid. A concentration dependent API release (first order kinetics) was observed from all lipid-based formulations. The API release rate was affected by the lipid crystalline size, the wettability of the system and the type of solid dispersion of API in the lipid-based matrix.
Deep understanding of these parameters combined with the application of the novel and robust analytical tools such as NIR-HSI is necessary to pave the way of developing advanced lipid-based formulations with tailored API release.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


