肌肉损伤诱导的神经调节蛋白egfr信号可动员干细胞进行全身再生。
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
创伤部位附近祖细胞的激活是跨物种再生的共同特征,但在全身再生中负责这一步骤的保守信号机制仍未完全了解。利用Piwi+多能成体干细胞(新生细胞),在再生早期积累在截肢部位,对迈阿密霍夫斯氏虫进行全身再生。EGFR信号通路在控制后生动物的增殖、迁移、分化和细胞存活方面具有广泛的作用。通过候选RNAi筛选,我们确定了Hofstenia EGFR EGFR -1和Neuregulin nrg-1基因对芽孢形成至关重要。NRG-1和EGFR-1蛋白的结构预测表明这些因子直接相互作用。截肢损伤后6小时nrg-1在创面体壁肌细胞中被诱导表达,24小时定位到离体胚尖并持续数天,而egfr-1广泛表达,包括在肌肉和新生细胞中。nrg-1表达的早期阶段发生在伤口愈合修复的切口部位,而晚期则特异于胚泡形成。在nrg-1(RNAi)和egfr-1(RNAi)损害胚泡形成的条件下,动物仍然会对损伤进行最早的反应,以激活早期生长反应转录因子egr的表达。此外,nrg-1的RNAi仅在截肢后才会导致再生失败,这表明再生需要新的nrg-1表达。nrg-1(RNAi)和egfr-1(RNAi)动物具有Piwi+和H3P+有丝分裂新细胞,这些细胞在截肢后正常增殖,但这些细胞不能在伤口部位聚集。因此,肌肉是损伤后神经调节因子egfr信号的来源,也是霍夫斯坦菌动员增殖祖细胞使胚壁生长以实现全身再生所必需的。这些结果表明,在5.5亿年的进化过程中,涡虫和恐龙之间对肌肉信号的功能需求是相同的,以实现再生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
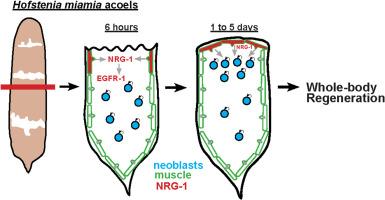
Injury-induced Neuregulin-EGFR signaling from muscle mobilizes stem cells for whole-body regeneration in acoels
The activation of progenitor cells near wound sites is a common feature of regeneration across species, but the conserved signaling mechanisms responsible for this step in whole-body regeneration are still incompletely understood. The acoel Hofstenia miamia undergoes whole-body regeneration using Piwi + pluripotent adult stem cells (neoblasts) that accumulate at amputation sites early in regeneration. The EGFR signaling pathway has broad roles in controlling proliferation, migration, differentiation, and cell survival across metazoans. Using candidate RNAi screening, we identify the Hofstenia EGFR egfr-1 and Neuregulin nrg-1 genes as essential for blastema formation. Structure prediction of NRG-1 and EGFR-1 proteins suggests these factors interact directly. After amputation injuries, nrg-1 expression is induced in body-wall muscle cells at the wound site by 6 h and localizes to the tip of the outgrowing blastema by 24 h and sustains for several days, while egfr-1 is broadly expressed, including in muscle and neoblasts. The early phase of nrg-1 expression occurs at incision sites that repair through wound healing while the late phase is specific to blastema formation. Under nrg-1(RNAi) and egfr-1(RNAi) conditions that impair blastema formation, animals still undergo the earliest responses to injury to activate expression of the Early Growth Response transcription factor egr. In addition, RNAi of nrg-1 only after amputation results in regeneration failure, indicating regeneration requires new nrg-1 expression. nrg-1(RNAi) and egfr-1(RNAi) animals possess Piwi+ and H3P + mitotic neoblasts which hyperproliferate normally after amputation, but these cells fail to accumulate at the wound site. Therefore, muscle provides a source for Neuregulin-EGFR signaling after injury and is necessary for the mobilization of proliferative progenitors to enable blastema outgrowth for whole-body regeneration in Hofstenia. These results indicate a shared functional requirement for muscle signaling to enable regeneration between planarians and acoels across 550 million years of evolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


