氧化锗纳米颗粒对锂离子导电NaCMC/PVA纳米复合膜电导率的影响
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究考察了纳米GeO2对溶液铸造法制备的NaCMC/PVA/ lib基固体聚合物电解质的离子电导率和结构性能的影响。XRD分析表明,随着GeO2含量的增加,非晶态特性增强。FTIR光谱证实了GeO2 NPs与聚合物盐基质之间的相互作用。SEM和EDX分析研究了其形貌和元素组成。紫外可见光谱显示,添加GeO2后,吸收边红移,带隙从5.70 eV减小到5.50 eV。阻抗谱(100 Hz - 5 MHz)表明离子电导率提高,符合Jonscher的通用幂定律,在5 wt%的GeO2下,最大值为(1.13±0.02)× 10−5 S cm−1。LSV确认电化学稳定性高达2.7 V。介电研究表明,由于界面极化,介电常数增加。转移数测量是利用极化技术评价固体聚合物电解质中离子载流子和电子载流子的相对贡献。这些结果突出了geo2分散NaCMC/PVA/LiBr薄膜在储能应用中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
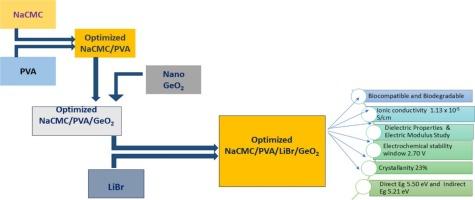
Influence of Germanium oxide nanoparticles on the electrical conductivity of Li-ion conducting NaCMC/PVA nanocomposite films
This study examines the influence of GeO2 nanoparticles (NPs) on the ionic conductivity and structural properties of NaCMC/PVA/LiBr-based solid polymer electrolytes synthesized via solution casting technique. XRD analysis revealed enhanced amorphous character with increasing GeO2 content. FTIR spectra confirmed interactions between GeO2 NPs and the polymer-salt matrix. SEM and EDX analyses were used to investigate morphology and elemental composition. UV–Vis spectroscopy showed a redshift in absorption edge and a bandgap reduction from 5.70 to 5.50 eV with GeO2 addition. Impedance spectroscopy (100 Hz - 5 MHz) indicated improved ionic conductivity, following Jonscher's universal power law, with a maximum value of (1.13 ± 0.02) × 10−5 S cm−1 at 5 wt% GeO2. LSV confirmed electrochemical stability up to 2.7 V. Dielectric studies revealed an increase in permittivity due to interfacial polarization. Transference number measurement evaluates the relative contributions of ionic and electronic charge carriers in solid polymer electrolytes using polarization technique. These results highlight the potential of GeO2-dispersed NaCMC/PVA/LiBr films for energy storage applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


