酸性蚀刻耦合配位组装原位合成鱼骨状Cu(II)-植酸聚合物,用于微rna -let 7a的电化学生物传感
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
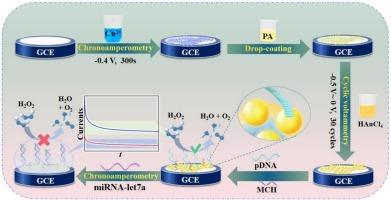
构建一种简便、高灵敏度的微rna电化学生物传感器对临床诊断具有重要意义。在本研究中,通过原位酸性蚀刻耦合配位组装,开发了鱼骨状Cu@Cu(II)-植酸(Cu@Cu(II)-PA)聚合物的界面。在进一步修饰电沉积金纳米颗粒(AuNPs)后,该界面作为多纳米复合材料用于构建miRNA-let 7a电化学生物传感器。首先,通过电沉积在玻碳电极(GCE)上制备一层金属铜。然后,将酸性PA溶液滴在电极上,化学蚀刻金属铜,生成的Cu(II)离子通过配位组装与PA反应形成Cu@Cu(II)-PA聚合物。随后,在Cu@Cu(II)-PA上电合成一层AuNPs,作为固定pDNA的基质。电化学分析表明,AuNP/Cu@Cu(II)-PA复合材料对H2O2具有良好的电催化活性,模拟过氧化物酶。将固定在电极表面的pDNA与目标miRNA-let 7a杂交,可以显著抑制AuNP/Cu@Cu(II)-PA的纳米酶活性,从而实现基于催化信号变化的miRNA-let 7a检测。分析表明,靶miRNA-let 7a可以在1.0 fM到10 nM的宽浓度范围内分析,检测限低至0.4 fM。此外,该生物传感器在检测真实血清样本中的miRNA-let 7a方面表现出令人称赞的性能,突出了其在早期疾病诊断中的临床应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acidic etching coupled with coordination assembly for in situ synthesis of fishbone-shaped Cu(II)-phytic acid polymer for electrochemical biosensing of microRNA-let 7a
The construction of a facile and highly sensitive electrochemical biosensor for microRNA detection has significant implications for clinical diagnostics. In this study, an interface integrating a fishbone-shaped Cu@Cu(II)-phytic acid (Cu@Cu(II)-PA) polymer was developed through in situ acidic etching coupled with coordination assembly. After further modification of electrodeposited gold nanoparticles (AuNPs), this interface served as a multiple-nanocomposite for the construction of an electrochemical biosensor for miRNA-let 7a. First, a layer of metallic copper was prepared on a glassy carbon electrode (GCE) through electrodeposition. Followed by, the acidic PA solution was dropped on the electrode, from which the metallic copper was chemically etched and the generated Cu(II) ions were reacted with PA to form Cu@Cu(II)-PA polymer through the coordination assembly. Subsequently, a layer of AuNPs was electrosynthesized on the Cu@Cu(II)-PA, acting as the matrix for pDNA immobilization. Electrochemical assays indicate that the AuNP/Cu@Cu(II)-PA composite exhibits excellent electrocatalytic activity towards H2O2, mimicking peroxidase. The hybridization of pDNA immobilized on the electrode surface with the target miRNA-let 7a significantly inhibits the nanozyme activity of AuNP/Cu@Cu(II)-PA, thereby enabling the detection of miRNA-let 7a based on catalytic signal variation. The analytical assay demonstrates that the target miRNA-let 7a can be analyzed over a wide concentration range from 1.0 fM to 10 nM with a detection limit as low as 0.4 fM. Furthermore, the biosensor exhibits commendable performance in the detection of miRNA-let 7a in real serum samples, highlighting its potential for clinical applications in early disease diagnosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


