内生真菌青霉菌(Penicillium sp. KYG-9)中的一种新型吲哚二萜
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从青霉菌KYG-9中鉴定了一种新的吲哚二萜根瘤素A(1)和一种已知的类似物(2)。通过全面的光谱分析,包括高分辨率电喷雾电离质谱(HR-ESI-MS)、x射线晶体学和电子圆二色性(ECD)计算,完成了结构解析。Penrhizovarin A(1)是第一个报道的吲哚二萜,其特点是具有前所未有的f环裂解和新型的4/6/6/6/ 6 - 6/6/6五环支架,其中包含罕见的二恶双环[3.3.1]壬烷单元。首次获得了2的单晶,并通过x射线单晶衍射进一步证实了其平面结构和立体化学性质。体外生物测定显示,penrhizovarin A对所有被测试的人类癌细胞系都表现出强大的细胞毒性,对HL-60(白血病)、A-549(肺癌)和ws -480(结肠癌)细胞系的活性尤其显著,超过了阳性对照顺铂的活性。此外,penrhizovarin A具有显著的抗真菌活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
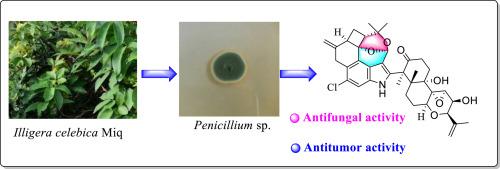
Penrhizovarin A, a novel indole diterpene from endophytic fungi Penicillium sp. KYG-9
One novel indole diterpene penrhizovarin A (1) and one known analogue (2), were characterized from Penicillium sp. KYG-9. Structural elucidation was accomplished through comprehensive spectroscopic analyses, including high-resolution electrospray ionization mass spectrometry (HR-ESI-MS), X-ray crystallography, and electronic circular dichroism (ECD) calculations. Penrhizovarin A (1) is the first reported indole diterpene characterized by both an unprecedented F-ring cleavage and a novel 4/6/6/6/5–6/6/6 pentacyclic scaffold incorporating a rare dioxabicyclo[3.3.1]nonane unit. The single crystal of 2 was obtained for the first time, and its planar structure and stereochemistry were further confirmed by X-ray single crystal diffraction. In vitro bioassays revealed that penrhizovarin A exhibited potent cytotoxicity against all tested human cancer cell lines, with particularly notable activity against HL-60 (leukemia), A-549 (lung cancer), and SW-480 (colon cancer) cell lines, surpassing the activity of the positive control cisplatin. Additionally, penrhizovarin A demonstrated significant antifungal activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


