从实验室到生物学:揭示跨鼓室药物传递的新型brijo体的效率
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-06
DOI:10.1016/j.jddst.2025.107493
引用次数: 0
摘要
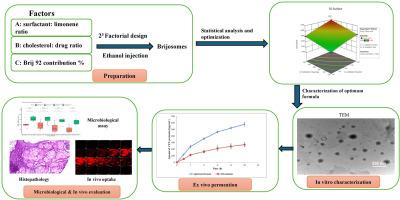
急性中耳炎(AOM)是一种常见的中耳感染,影响了美国近三分之一的儿童。本研究着重于通过将环丙沙星(CFX)包埋在brijosoms(一种新型纳米囊泡系统,用于改善耳局部治疗)中来增强环丙沙星(CFX)的非侵入性经鼓室输送。采用乙醇注射法制备Brijosomes,并通过23全因子设计对其进行优化,考察了表面活性剂:柠檬烯比、胆固醇:药物比和brij92浓度的影响。评估的关键参数包括捕获效率(EE%)、粒径(PS)、多分散性指数(PDI)和ζ电位(ZP)。通过Design Expert®软件选择的优化配方显示出较高的理想值0.979,EE%为91.68%,PS为196.85 nm, ZP为−25.45 mV。表征研究表明,优化后的Brijosomal分子式具有球形结构,具有双相体外释放特性,在5±3°C下保持稳定3个月。体外渗透研究表明,与CFX溶液相比,其渗透性和通量均优于CFX溶液,增强比为2.15倍。微生物学评价证实优化后的配方具有较好的抗菌和抗生物膜活性。此外,共聚焦激光扫描显微镜(CLSM)证实了更深的穿透,与离体研究结果一致。组织病理学检查证实该制剂用于耳外用是安全的。这些发现共同强调了cfx负载Brijosomes作为AOM治疗有效的非侵入性药物传递系统的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
From bench to biology: Unraveling the efficiency of novel brijosomes for trans-tympanic drug delivery
Acute otitis media (AOM) is a prevalent middle ear infection, affecting nearly one-third of children in the U.S. This study focused on enhancing non-invasive trans-tympanic delivery of ciprofloxacin (CFX) by encapsulating it within Brijosomes, a novel nano-vesicular system for improved ototopical treatment. Brijosomes were formulated using the ethanol injection technique and optimized through a 23 full factorial design, investigating the effects of surfactant: limonene ratio, cholesterol: drug ratio, and Brij 92 concentration. Key parameters evaluated included entrapment efficiency (EE%), particle size (PS), polydispersity index (PDI), and zeta potential (ZP). The optimized formulation, selected via Design Expert® software, demonstrated a high desirability value of 0.979, with EE% of 91.68 %, PS of 196.85 nm, and ZP of −25.45 mV. Characterization studies revealed that the optimized Brijosomal formula had a spherical morphology, exhibited a bi-phasic in vitro release profile, and remained stable for three months at 5 ± 3 °C. Ex vivo permeation studies demonstrated superior penetration and flux compared to CFX solution, with a 2.15-fold enhancement ratio. Microbiological assessments confirmed improved antibacterial and antibiofilm activities of the optimized formulation. Additionally, confocal laser scanning microscopy (CLSM) validated deeper penetration, consistent with the ex vivo findings. Histopathological examination confirmed the formulation's safety for ototopical application. These findings collectively emphasize the potential of CFX-loaded Brijosomes as an effective, non-invasive drug delivery system for AOM treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


