利用Argonaute 2作为siRNA的创新递送系统治疗胶质母细胞瘤
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-04
DOI:10.1016/j.jddst.2025.107459
引用次数: 0
摘要
通过小干扰RNA (siRNA)进行RNA干扰为治疗胶质母细胞瘤(GB)提供了有希望的潜力,胶质母细胞瘤是最常见和侵袭性的原发性脑肿瘤。虽然局部siRNA递送可以绕过血脑屏障并获得高药物浓度,但开发有效的递送系统仍然存在挑战,以确保siRNA的稳定性、有效的细胞摄取、内溶酶体逃逸和长期的脑可用性。Argonaute-2 (Ago2)蛋白是RNA干扰机制的核心组成部分,可自然结合siRNA并保护其免受降解。Ago2也存在于生物液体中,如血液,以及细胞外囊泡,包括外泌体和微囊泡,使其成为siRNA递送的潜在天然载体。本研究探索了Ago2作为siRNA的天然载体,在纳米颗粒递送系统中增强siRNA的转运和基因沉默。中性聚合物siRNA/ ago2负载纳米颗粒(NPs)的尺寸约为300 nm,具有很高的包封效率(蛋白质为85.1%,siRNA为57.5%)。在稳定表达荧光素酶(U87MG Bml1)的U87MG GB细胞中进行的体外测试表明,与游离siRNA/Ago2复合物相比,负载siRNA/Ago2的NPs有效地降低了荧光素酶的表达,与混乱的siRNA复合物相比,在48小时内没有明显的细胞毒性。有趣的是,将外源Ago2引入细胞意外上调了荧光素酶的表达,这表明Ago2还可能影响全局基因表达,而不仅仅是在siRNA介导的沉默中起作用。这些发现突出了siRNA/Ago2负载NPs在GB中局部siRNA递送的潜力,并表明Ago2除了增强siRNA活性外,还可以在基因表达中发挥更广泛的调节作用。需要进一步研究Ago2对基因调控的影响及其作为GB治疗工具的潜力,特别是与生物相容性水凝胶联合持续递送。本文章由计算机程序翻译,如有差异,请以英文原文为准。
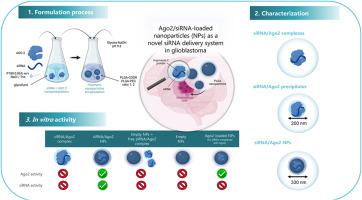
Harnessing Argonaute 2 as an innovative delivery system for siRNA in glioblastoma treatment
RNA interference via small interfering RNA (siRNA) offers promising potential for treating glioblastoma (GB), the most common and aggressive primary brain tumor. While local siRNA delivery could bypass the blood-brain barrier and achieve high drug concentrations, challenges remain in developing effective delivery systems that ensure siRNA stability, efficient cellular uptake, endolysosomal escape, and long-term brain availability. The Argonaute-2 (Ago2) protein, a core component of the RNA interference machinery, naturally binds siRNA and protects it from degradation. Ago2 is also found in biological fluids, such as blood, and in extracellular vesicles, including exosomes and microvesicles, making it a potential natural vector for siRNA delivery.
This study explores Ago2 as a natural vector for siRNA in nanoparticle-based delivery systems to enhance siRNA transport and gene silencing in GB. Neutral polymeric siRNA/Ago2-loaded nanoparticles (NPs), around 300 nm in size, were formulated with high encapsulation efficiencies (85.1 % for protein and 57.5 % for siRNA). In vitro testing in U87MG GB cells stably expressing luciferase (U87MG Bml1) showed that, in contrast to free siRNA/Ago2 complexes, siRNA/Ago2-loaded NPs effectively reduced luciferase expression compared to scramble siRNA formulations, with no significant cytotoxicity at 48 h. Interestingly, the introduction of exogenous Ago2 into cells unexpectedly upregulated luciferase expression, suggesting that Ago2 may also influence global gene expression beyond its role in siRNA-mediated silencing.
These findings highlight the potential of siRNA/Ago2-loaded NPs for local siRNA delivery in GB and suggest that Ago2 could play a broader regulatory role in gene expression, in addition to enhancing siRNA activity. Further investigation is needed to explore Ago2's impact on gene regulation and its potential as a therapeutic tool in GB treatment, particularly in combination with biocompatible hydrogels for sustained delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


