利用基于超声的深度学习模型改进早期HCC的风险分层和检测
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
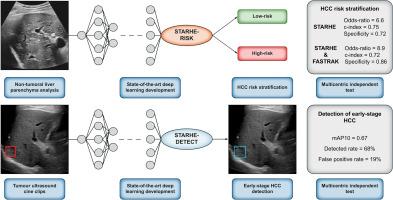
背景:肝细胞癌(HCC)的监测方案并不理想。我们旨在设计一个基于超声的深度学习模型,用于代偿性晚期慢性肝病(cACLD)患者的HCC风险分层(STARHE-RISK)和早期HCC检测(STARHE-DETECT)。方法:这项前瞻性多中心研究纳入了403名所有原因的成年cACLD患者,他们参加了一个至少6个月的无HCC病史的监测项目。STARHE-RISK对非肿瘤性肝组织的超声影像片段进行训练,分为两类:病例(n = 152例早期HCC患者;137/152例[82%]男性;中位年龄63岁)和对照组(n = 170例纳入时和随后1年随访期间未发生HCC的患者;120/170例[71%]男性;中位年龄69岁)。STARHE-DETECT对肿瘤超声短片进行训练。根据潜在混杂因素对训练/验证和测试集进行分层,根据样本量计算将两组平衡的50例患者分配到独立测试集。统计分析包括分类和检测指标。结果starhe - risk在测试集中获得了良好的预测性能,准确率为0.72 (95% CI 0.57-0.84),优势比为6.6 (95% CI 1.9-22.7; p = 0.003)。STARHE-RISK和FASTRAK评分(一种多病因HCC风险分层评分)的结合,在预测HCC发展高风险患者方面具有更高的特异性(0.86 [95% CI 0.65-0.97])和优势比(8.9 [95% CI 2.1-38.3; p = 0.004])。STARHE-DETECT检测早期HCC的mAP10为0.67,灵敏度为0.68 (95% CI 0.47-0.85),特异性为0.82 (95% CI 0.69-0.91)。结论starhe - risk和STARHE-DETECT分别在HCC风险分层和早期HCC检测方面取得了较好的效果。它们可能成为有价值的监控工具,并为基于风险的个性化监控项目铺平道路。影响和意义starhe - risk是一种可靠的基于超声的深度学习模型,用于代偿性晚期慢性肝病患者的肝细胞癌(HCC)风险分层,并可与临床和血液参数的互补评分相关联。STARHE-DETECT可以成为肝癌监测中放射科医生和超声医师视觉评估的补充工具。这两种模型都是基于简单和易于执行的超声电影剪辑采集。这项研究为基于风险的个性化监测计划铺平了道路,该计划最终将不依赖于单一测试,而是依赖于混合临床,生物学和放射学数据的方法组合。临床试验注册本研究已在ClinicalTrials.gov注册(NCT04802954)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Improving risk stratification and detection of early HCC using ultrasound-based deep learning models
Background & Aims
Hepatocellular carcinoma (HCC) surveillance programs are suboptimal. We aimed to design an ultrasound-based deep learning model for HCC risk stratification (STARHE-RISK) and early-stage HCC detection (STARHE-DETECT) in patients with compensated advanced chronic liver disease (cACLD).
Methods
This prospective multicentric study included 403 adult patients with cACLD of all causes enrolled in a surveillance program for at least 6 months without prior history of HCC. STARHE-RISK was trained on ultrasound cine clips of the non-tumoral liver parenchyma using two classes: cases (n = 152 patients with early-stage HCC; 137/152 [82%] male; median age 63 years) and controls (n = 170 patients without HCC at inclusion and during a subsequent 1-year follow-up; 120/170 [71%] male; median age 69 years). STARHE-DETECT was trained on tumour ultrasound cine clips. The training/validation and testing sets were stratified according to potential confounders, and 50 patients who were balanced in both groups were allocated to the independent testing set based on sample size calculation. Statistical analysis included classification and detection metrics.
Results
STARHE-RISK achieved good prediction performances in the testing set with a 0.72 accuracy (95% CI 0.57–0.84) and an odds ratio of 6.6 (95% CI 1.9–22.7; p = 0.003). The combination of STARHE-RISK and the FASTRAK score, a multi-aetiology HCC risk stratification score, achieved a higher specificity (0.86 [95% CI 0.65–0.97]) and odds ratio (8.9 [95% CI 2.1–38.3; p = 0.004]) for predicting a patient at high risk of HCC development. STARHE-DETECT achieved a 0.67 mAP10, a 0.68 sensitivity (95% CI 0.47–0.85), and a 0.82 specificity (95% CI 0.69–0.91) for detecting early-stage HCC.
Conclusions
STARHE-RISK and STARHE-DETECT achieved robust performances for HCC risk stratification and early-stage HCC detection, respectively. They could become valuable surveillance tools and pave the way for a risk-based personalised surveillance program.
Impact and implications
STARHE-RISK is a reliable ultrasound-based deep learning model for hepatocellular carcinoma (HCC) risk stratification in patients with compensated advanced chronic liver disease and can be associated with complementary scores integrating clinical and blood parameters. STARHE-DETECT could become a complementary tool to visual assessment for radiologists and sonographers in HCC surveillance. Both models are based on simple and easy-to-perform ultrasound cine clip acquisitions. This study paves the way for a risk-based personalised surveillance program that will not ultimately rely on a single test but rather on a combination of approaches mixing clinical, biological, and radiological data.
Clinical Trials Registration
The study is registered at ClinicalTrials.gov (NCT04802954).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


