氧空位诱导的具有增强磁性的mgfe层状双氢氧化物通过磁分离有效去除污染物
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
磁性纳米材料由于其易于分离和可重复使用,长期以来被认为是环境修复的有希望的候选者。然而,在单一材料中实现强磁性和高吸附能力仍然是一个重大挑战,特别是在平衡磁性和结构完整性方面。在本研究中,采用常规共沉淀法合成了含有Mg-Fe成分的层状双氢氧化物(LDHs),然后用硼氢化钠(LDH-VO)进行化学处理。原始LDH和LDH- vo均表现出结晶良好的无杂质辉钼矿结构,保持了相似的形貌。为了检查局部电子结构的变化,进行了一系列的光谱分析。观察到配体到金属跃迁、吸收红移和超色效应的增加,表明氧空位的发展。LDH- vo的带隙比LDH窄,可能是化学处理后电子结构发生了改变。此外,LDH-VO表现出较低的对称序和Fe的部分还原,反映了电子和局部结构的变化。LDH- vo比原始LDH含有更多的缺陷氧位点。磁测量表明,由于这些局部变化,LDH-VO的磁性能发生了变化。在300 K和77 K时,原始LDH表现出极低磁化率的顺磁性行为,而LDH- vo在300 K时表现出超顺磁性,在77 K时表现出铁/铁磁性。这归因于氧空位破坏了通常支持反铁磁相互作用的Fe-O-Fe超交换途径。磁性能的增强是由结构缺陷和铁的部分还原引起的。此外,缺陷工程增加了比表面积(LDH的SBET = 65.644 m2/g, LDH- vo的SBET = 90.592 m2/g),促进了表面非均匀性(LDH的n = 1.50, LDH- vo的n = 3.47)。LDH-VO通过磁分离表现出高效的污染物去除(513 mg/g),突出了缺陷位点在增强磁响应和静电吸附相互作用方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
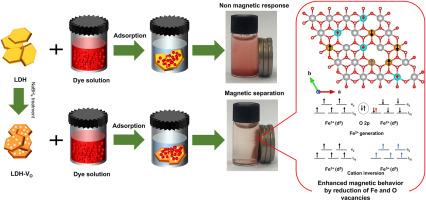
Oxygen vacancy-induced MgFe-layered double hydroxide with enhanced magnetism for efficient pollutant removal via magnetic separation
Magnetic nanomaterials have long been considered promising candidates for environmental remediation due to their ease of separation and reusability. However, achieving both strong magnetism and high adsorption capacity in a single material has remained a significant challenge, particularly in balancing magnetic properties with structural integrity. In this study, layered double hydroxides (LDHs) with Mg-Fe composition were synthesized using conventional coprecipitation and subsequently chemically treated with sodium borohydride (LDH-VO). Both pristine LDH and LDH-VO exhibited well-crystallized pyroaurite structures without impurities, maintaining comparable morphology. To examine changes in the local electronic configuration, a series of spectroscopic analyses were performed. An increased ligand-to-metal transition, redshift of absorption, and hyperchromic effect were observed, indicating the development of oxygen vacancies. The bandgap of LDH-VO was found to be narrower than that of LDH, likely due to the modified electronic structure following chemical treatment. Additionally, LDH-VO displayed a lower symmetry order and partial reduction in Fe, reflecting changes in the electronic and local structures. LDH-VO contained more defective oxygen sites than pristine LDH. Magnetic measurements demonstrated altered magnetic properties in LDH-VO due to these local changes. At 300 K and 77 K, pristine LDH exhibited paramagnetic behavior with very low magnetic susceptibility, whereas LDH-VO showed superparamagnetism at 300 K and ferro/ferrimagnetism at 77 K. It was attributed to oxygen vacancies disrupting the Fe–O–Fe super-exchange pathway that typically supports antiferromagnetic interactions. The enhanced magnetic properties were attributed to the induced structural defects and partial reduction of Fe. Additionally, defect engineering increased the specific surface area (SBET = 65.644 m2/g for LDH, and SBET = 90.592 m2/g for LDH-VO) and promoted surface heterogeneity (n = 1.50 for LDH, and n = 3.47 for LDH-VO). The LDH-VO exhibited efficient pollutant removal (513 mg/g) through magnetic separation, highlighting the crucial role of defect sites in enhancing both magnetic response and adsorption interactions beyond electrostatic forces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Nano
Multiple-
CiteScore
11.30
自引率
3.90%
发文量
130
审稿时长
31 days
期刊介绍:
Materials Today Nano is a multidisciplinary journal dedicated to nanoscience and nanotechnology. The journal aims to showcase the latest advances in nanoscience and provide a platform for discussing new concepts and applications. With rigorous peer review, rapid decisions, and high visibility, Materials Today Nano offers authors the opportunity to publish comprehensive articles, short communications, and reviews on a wide range of topics in nanoscience. The editors welcome comprehensive articles, short communications and reviews on topics including but not limited to:
Nanoscale synthesis and assembly
Nanoscale characterization
Nanoscale fabrication
Nanoelectronics and molecular electronics
Nanomedicine
Nanomechanics
Nanosensors
Nanophotonics
Nanocomposites
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


