单晶和陶瓷(NH4)3H(SO4)2中的常规和逆机械热效应
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
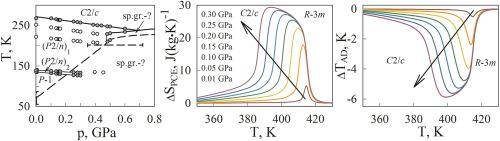
对单晶和陶瓷(NH4)3H(SO4)2在大气压下经历一系列结构变换的热容量、热膨胀、对流体静压的敏感性以及机械热效应进行了详细的研究:R-3m↔(C2/c)↔(P2/n)1↔(P2/n)2↔P-1。观察到异常对陶瓷样品变形的显著影响,特别是在一阶变换附近,而两个样品的异常熵的行为和值是令人满意的一致。本文首次对T - p相图的低温相变区域进行了实验研究。实测的体积压系数与计算的体积压系数有很好的对应关系。利用熵温相图对不同静水/单轴压力下的气压(BCE)和压电(PCE)热效应进行了对比分析。逆BCE是所研究的所有相变的特征,这是由于它们在静水压力下温度降低引起的。由于晶体相的对称性较低,(NH4)3H(SO4)2在热膨胀中表现出很强的各向异性,这导致了线性压系数的值和符号的差异,从而导致了与各种晶体轴相关的常规和逆PCE。分析了单晶和陶瓷(NH4)3H(SO4)2的热参数,并与其他硫酸铵衍生物进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Conventional and inverse mechanocaloric effects in single-crystal and ceramic (NH4)3H(SO4)2
Detailed studies of heat capacity, thermal expansion, sensitivity to hydrostatic pressure, as well as mechanocaloric effects were carried out for single-crystal and ceramic (NH4)3H(SO4)2 undergoing a number of structural transformations at atmospheric pressure: R-3m ↔ (C2/c) ↔ (P2/n)1 ↔ (P2/n)2 ↔ P-1. A significant smearing of the anomalous contribution to the deformation of the ceramic sample was observed, especially near first order transformations, while the behavior and values of the anomalous entropy are in satisfactory agreement for both samples. For the first time, the region of the T – p phase diagram, including low temperature phase transitions, was experimentally investigated. A good correspondence was found between the measured and calculated volumetric baric coefficients. A comparative analysis of the baro(BCE)- and piezo(PCE)-caloric effects was carried out using entropy–temperature phase diagrams at various hydrostatic/uniaxial pressures. Inverse BCE is characteristic of all studied phase transitions, which is caused by a decrease in their temperatures under hydrostatic pressure. Due to rather low symmetry of the crystalline phases, (NH4)3H(SO4)2 demonstrates a strong anisotropy in the thermal expansion which leads in turn to the difference in the values and sign of the linear baric coefficients and, as a result, to conventional and inverse PCE associated with the various crystallographic axes. The caloric parameters of single-crystal and ceramic (NH4)3H(SO4)2 are analyzed in comparison with some other derivatives of ammonium sulphate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


