基于含氰有机催化剂的二氧化碳电还原制乙烯
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
电化学CO2还原反应(eCO2RR)产生气态C2+产物,如乙烯(C2H4),是缓解温室效应的可持续策略。受到有机化学中氰基(-C≡N)对C-C偶联的促进作用的启发,已经发现几种含氰的有机催化剂能够在eCO2RR过程中以-C≡N为活性中心直接将CO2转化为C2H4。在kco3溶液中部分加氢后,C2H4对无金属双氰胺(DCD)的选择性达到27.6%。此外,当与取向铜晶体耦合时,其选择性可进一步提高到57.7%。实验和计算结果共同揭示了Cu{100}和DCD之间的电荷重分布促进了氰基的部分氢化,为c≡N上CO2还原的能量垒降低奠定了基础。本研究突破了传统金属/金属氧化物基催化剂的局限,采用含氰有机催化剂直接生成C2+产物,扩展了eCO2RR催化剂库。此外,本研究阐明了电荷重分配和氰基加氢在降低反应障碍中的作用,为新型有机催化剂的设计提供了基础指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
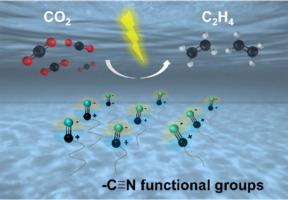
Carbon dioxide electroreduction to ethylene based on cyano-containing organocatalysts
The electrochemical CO2 reduction reaction (eCO2RR), producing gaseous C2+ products such as ethylene (C2H4), represents a sustainable strategy to mitigate the greenhouse effect. Inspired by the promotion effect of the cyano group (–C≡N) for C–C coupling in organic chemistry, several cyano-containing organocatalysts have been found to be capable of directly converting CO2 into C2H4 with –C≡N as the active center during the eCO2RR. The selectivity of C2H4 for the representative catalyst, metal-free dicyandiamide (DCD), reached 27.6 % after partial hydrogenation in KHCO3 solution. In addition, its selectivity can be further improved to 57.7 % when coupled with oriented Cu crystals. The experimental and computational results collectively reveal that charge redistribution between Cu{100} and DCD promotes the partial hydrogenation of the cyano group and lays the foundation for the reduced energy barrier for the CO2 reduction on –C≡N. This study breaks the limitations of traditional metal/metal oxide-based catalysts by using cyano-containing organocatalysts for direct C2+ product generation, expanding the eCO2RR catalyst library. In addition, this research elucidates the role of charge redistribution and cyano group hydrogenation in lowering reaction barriers, providing fundamental guidance for the design of new organocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


