世界卫生组织癌症临床试验的全球概况
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
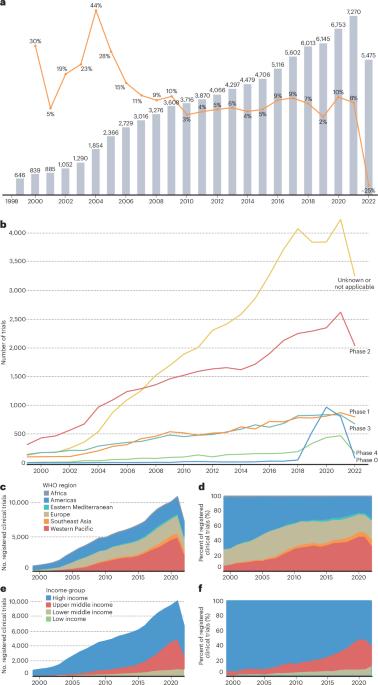
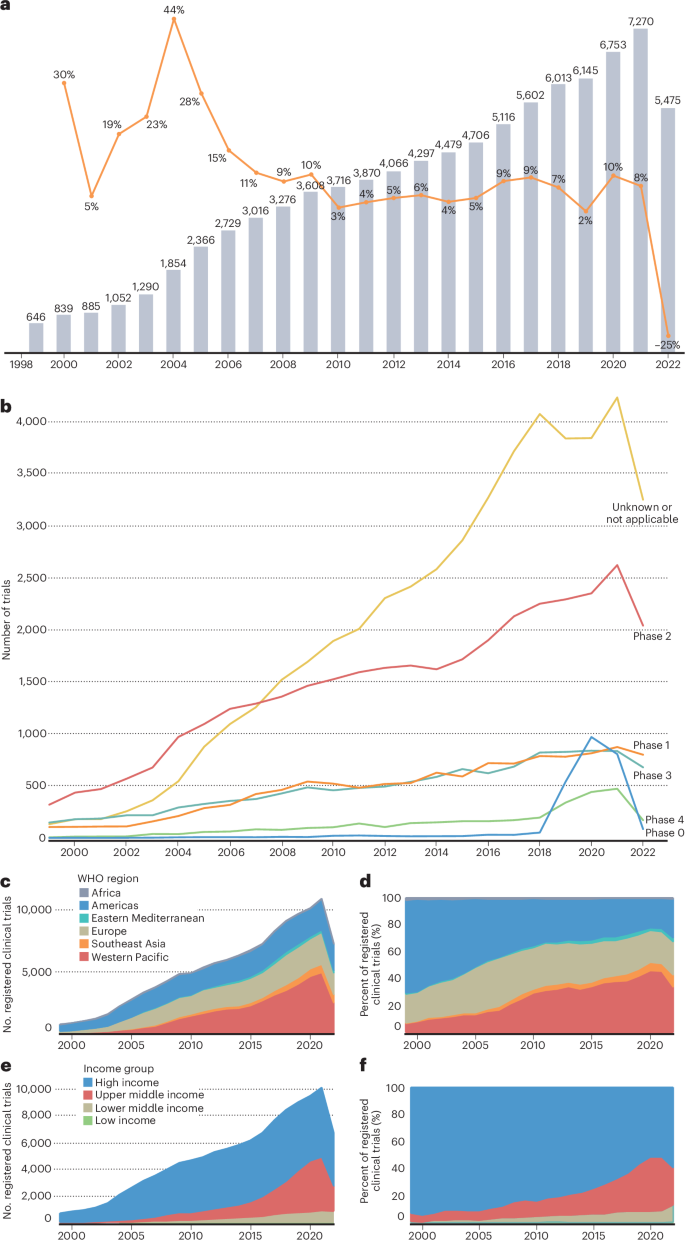
临床试验对推进癌症控制至关重要,但在全球范围内,临床试验的获取和参与仍然不平等。世界卫生组织(世卫组织)建立了国际临床试验注册平台(ICTRP),以使所有参与保健决策的人能够全面了解介入性临床研究,并确定在全球一级公平参与的可行目标。对1999年至2022年12月期间在世卫组织ICTRP注册的89069项全球癌症临床试验的回顾显示,癌症临床试验格局由高收入国家主导,并侧重于药物干预,跨国合作仅占招募试验的3%。一些最致命的癌症,包括肝癌、胃癌、胰腺癌和宫颈癌,一直没有出现在研究最多的癌症类型中,尤其是在非洲和东南亚。在本综述中,我们总结了世卫组织全球景观综述的主要发现,并讨论了根据这些数据采取行动的战略,这些数据为政策、实践和投资决策提供了关键的经验证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The WHO global landscape of cancer clinical trials
Clinical trials are essential to advancing cancer control, yet access and participation remain unequal globally. The World Health Organization (WHO) established the International Clinical Trials Registry Platform (ICTRP) to enable a complete view of interventional clinical research for all those involved in healthcare decision-making and to identify actionable goals to equitable participation at the global level. A review of 89,069 global cancer clinical trials registered in the WHO ICTRP between 1999 and December 2022 revealed a cancer clinical trial landscape dominated by high-income countries and focused on pharmacological interventions, with multinational collaboration limited to only 3% of recruiting trials. Several of the deadliest cancers, including liver, stomach, pancreas and cervical cancer, were consistently missing from the top most-studied cancer types, particularly in Africa and Southeast Asia. In this Review, we summarize the key findings of the WHO global landscape review and discuss strategies to act on these data, which provide critical empirical evidence to inform policy, practice and investment decisions. This Review of the WHO’s International Clinical Trials Registry Platform presents a snapshot of the global cancer trial landscape and provides critical empirical evidence to inform policy, practice and investment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


