沿海沉积物中的短链PFAS: pfbs驱动的抗菌素耐药性和病原体风险
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
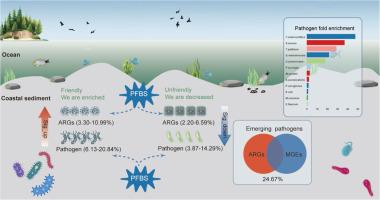
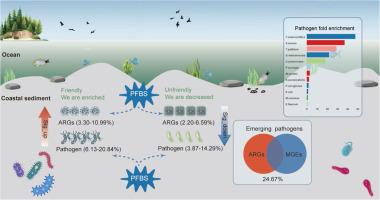
沿海沉积物是全氟丁烷磺酸(PFBS)、抗生素抗性体和病原体等新出现污染物的储存库,对海洋生态系统和公众健康构成风险。在这里,我们通过宏基因组学研究PFBS对沉积物中抗菌素耐药性和病原体动力学的影响。研究结果表明,沿海沉积物中含有多种抗生素耐药基因(ARGs)、移动遗传元件(MGEs)和细菌病原体,PFBS暴露显著改变了它们的丰度和组成。PFBS暴露使ARGs(34.44% ~ 51.11%)和MGEs(42.96% ~ 52.96%)升高。重要的是,高风险vanY (vanB培养菌)(1.37倍)和vanYG1(1.21倍)显著富集。此外,在检测到的1190种病原体中,67种新出现的病原体在所有28个样本中广泛共享。值得注意的是,在pfbs暴露的沉积物中,常见的苍白t菌(9.69倍)和肺炎s菌(3.82倍)表现出最明显的增加。进一步分析发现,24.67%的病原菌同时携带ARGs和MGEs,增强了耐药性传播和病原菌毒力。值得注意的是,新出现的耻垢分枝杆菌和假结核分枝杆菌是主要的高风险变种(vanM集群),对沿海生态系统构成严重的抗微生物威胁。这些结果强调PFBS是沿海沉积物中抗菌药物耐药性和致病菌增殖的关键驱动因素,强调迫切需要进一步了解短链PFAS对海洋生态安全的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Short-chain PFAS in coastal sediments: PFBS-driven antimicrobial resistance and pathogen risks
Coastal sediments serve as reservoirs for emerging contaminants, like perfluorobutane sulfonic acid (PFBS), antibiotic resistomes, and pathogens, posing risks to marine ecosystems and public health. Here, we investigate the effects of PFBS on antimicrobial resistance and pathogen dynamics in sediments through metagenomics. Our findings show that coastal sediments contain various antibiotic resistance genes (ARGs), mobile genetic elements (MGEs), and bacterial pathogens, with PFBS exposure significantly altering their abundance and composition. PFBS exposure increased ARGs (34.44 %–51.11 %) and MGEs (42.96 %–52.96 %). Importantly, high-risk vanY (vanB culuster) (1.37-fold) and vanYG1 (1.21-fold), were considerably enriched. Additionally, among 1190 detected pathogens, 67 emerging pathogens were extensively shared across all 28 samples. Notably, prevalent T. pallidum (9.69-fold) and S. pneumoniae (3.82-fold) exhibited the most pronounce increases in PFBS-exposed sediments. Further analysis revealed that 24.67 % of the pathogens co-harbored both ARGs and MGEs, amplifying resistance dissemination and pathogen virulence. Remarkably, emerging M. smegmatis and Y. pseudotuberculosis, harboring the predominant high-risk vanY (vanM cluster), pose a critical antimicrobial threat to coastal ecosystems. These results underscore PFBS as a key driver of antimicrobial resistance and pathogenic proliferation in coastal sediments, highlighting the urgent need for further insight into the effects of short-chain PFAS on marine ecological security.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


