Durotaxis是肺纤维化和转移性胰腺癌的驱动因子和潜在治疗靶点
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
硬度趋向性,细胞沿硬度梯度迁移,与胚胎发育、组织修复和疾病有关。尽管有确凿的体外证据,但其在体内的作用在很大程度上仍是推测性的。在这里,我们证明了durotaxis在肺纤维化和转移性胰腺癌的小鼠模型中积极推动疾病进展。在肺纤维化中,硬原性引导成纤维细胞募集到损伤部位,在那里它们被机械激活成瘢痕形成的肌成纤维细胞。在胰腺癌中,肿瘤微环境的硬化诱导癌细胞的硬化性,促进转移扩散。从机制上讲,硬化性是由局灶黏着激酶(FAK) -paxillin相互作用介导的,这是一种机械感觉模块,通过YAP信号将僵硬线索与转录程序联系起来。为了从遗传学上探索这一点,我们产生了一只FAK-FATL994E敲入小鼠,它破坏了FAK-paxillin的结合,阻断了durotaxis并减轻了疾病的严重程度。FAK-paxillin与小分子JP-153相互作用的药理抑制模拟了这些作用。我们的研究结果证实了durotaxis在体内是一种疾病机制,并支持抗durotactic治疗作为治疗纤维化和癌症的潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
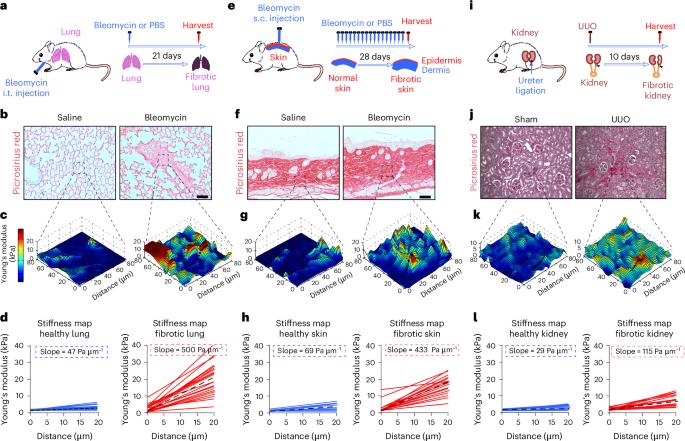
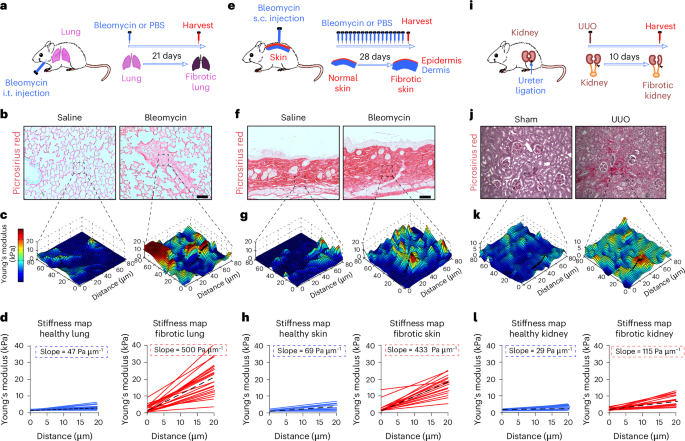
Durotaxis is a driver and potential therapeutic target in lung fibrosis and metastatic pancreatic cancer
Durotaxis, cell migration along stiffness gradients, is linked to embryonic development, tissue repair and disease. Despite solid in vitro evidence, its role in vivo remains largely speculative. Here we demonstrate that durotaxis actively drives disease progression in vivo in mouse models of lung fibrosis and metastatic pancreatic cancer. In lung fibrosis, durotaxis directs fibroblast recruitment to sites of injury, where they undergo mechano-activation into scar-forming myofibroblasts. In pancreatic cancer, stiffening of the tumour microenvironment induces durotaxis of cancer cells, promoting metastatic dissemination. Mechanistically, durotaxis is mediated by focal adhesion kinase (FAK)–paxillin interaction, a mechanosensory module that links stiffness cues to transcriptional programmes via YAP signalling. To probe this genetically, we generated a FAK-FATL994E knock-in mouse, which disrupts FAK–paxillin binding, blocks durotaxis and attenuates disease severity. Pharmacological inhibition of FAK–paxillin interaction with the small molecule JP-153 mimics these effects. Our findings establish durotaxis as a disease mechanism in vivo and support anti-durotactic therapy as a potential strategy for treating fibrosis and cancer. Al-Hilal, Chrysovergi et al. identify a role for durotaxis in lung fibrosis and metastatic pancreatic cancer, mediated by the FAK–paxillin mechanosensor complex, and demonstrate therapeutic targeting of this pathway using the small molecule JP-153.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


