不同的细胞因子和转录特征控制功能性T滤泡辅助细胞异质性
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
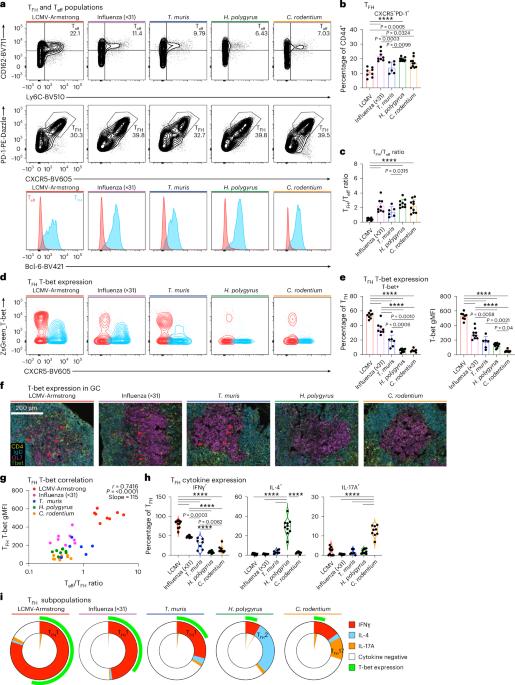
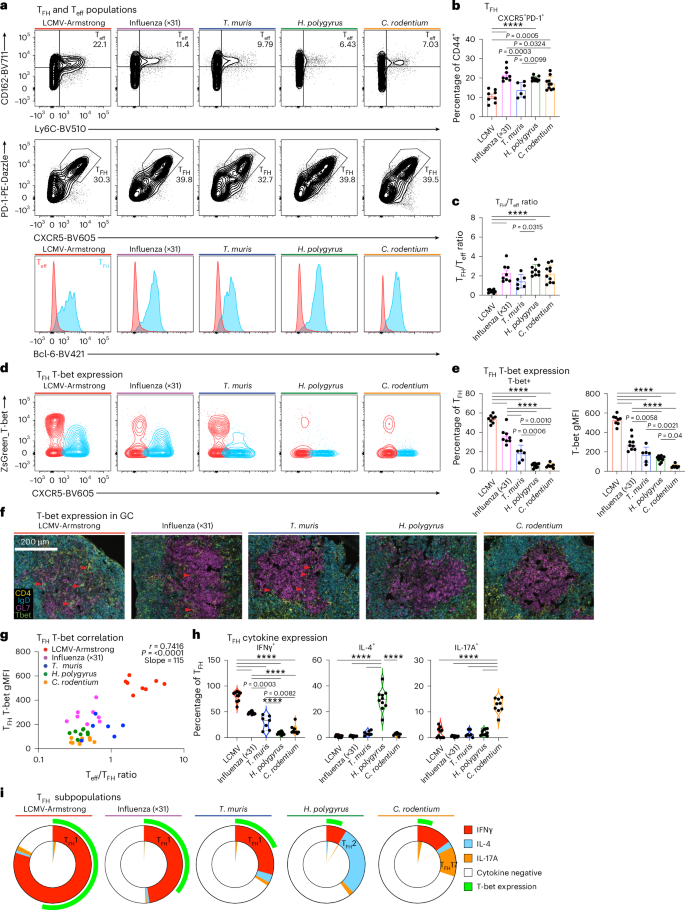
CD4+ T滤泡辅助细胞(TFH)支持针对多种病原体的量身定制的B细胞反应。为了揭示TFH表型的多样性,我们分析了小鼠TFH细胞对病毒、蠕虫和细菌感染的反应。我们确定了一个与CD4+ T滤泡调节细胞和效应细胞不同的核心TFH特征,并确定了塑造TFH功能的病原体特异性转录模块。细胞因子转录TFH编程表明,I型干扰素和TGFβ信号直接指示个体TFH表型,指导B细胞输出。细胞因子导向的TFH转录表型在人类生发中心内是共享的,但不同的TFH表型在供体之间和免疫挑战后或抗体介导的疾病中占主导地位。最后,我们确定了与不同TFH表型一致的新的细胞表面标记。因此,我们提供了人类和小鼠中TFH多样性的综合资源,以便在感染和疾病期间进行免疫监测,并为开发特定情况的疫苗提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Divergent cytokine and transcriptional signatures control functional T follicular helper cell heterogeneity
CD4+ T follicular helper (TFH) cells support tailored B cell responses against multiple classes of pathogens. To reveal how diverse TFH phenotypes are established, we profiled mouse TFH cells in response to viral, helminth and bacterial infection. We identified a core TFH signature that is distinct from CD4+ T follicular regulatory and effector cells and identified pathogen-specific transcriptional modules that shape TFH function. Cytokine-transcriptional TFH programming demonstrated that type I interferon and TGFβ signaling direct individual TFH phenotypes to instruct B cell output. Cytokine-directed TFH transcriptional phenotypes are shared within human germinal centers, but distinct TFH phenotypes dominate between donors and following immune challenge or in antibody-mediated disease. Finally, we identified new cell surface markers that align with distinct TFH phenotypes. Thus, we provide a comprehensive resource of TFH diversity in humans and mice to enable immune monitoring during infection and disease and to inform the development of context-specific vaccines. Dalit, Tan and colleagues provide a multiomic profile of T follicular helper (TFH) cells responses to diverse pathogens, revealing a blueprint for transcriptional flexibility and new tools to interrogate TFH heterogeneity in mice and humans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


