LaPO4晶体相在控制葡萄糖生成5-羟甲基糠醛的酸性位点中的关键作用
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
糖转化生产5-羟甲基糠醛(5-HMF)由于反应效率低、成本高而未能达到工业预期。本文采用水热法制备了一系列晶体结构可控、酸度可调的LaPO4催化剂。六方LaPO4-120催化剂在150℃下反应60 min,葡萄糖转化率达到99.9%,5-HMF收率达到90.1%。此外,六方LaPO4-120催化剂在转化果糖、葡萄糖、纤维素二糖、蔗糖和菊粉等多种糖类方面表现出良好的活性。一系列表征研究和DFT计算表明,六方LaPO4-120催化剂具有低配位的La位和丰富的沸石水,这些沸石水储存在开放和氧衬里的通道中,分别提供了Lewis酸位点和动态局部Brønsted酸环境,分别促进葡萄糖异构化和随后的脱水。提出了一种通过氢键进行质子协同转移的反应机理。该研究为低成本和多相催化剂的设计提供了见解,这些催化剂具有精细调节的Lewis和Brønsted酸位点,用于生产5-HMF。本文章由计算机程序翻译,如有差异,请以英文原文为准。
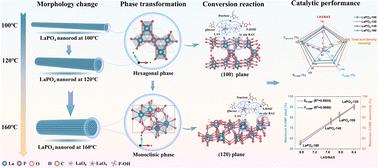
The critical role of crystal phases of LaPO4 in controlling the acidic sites for the production of 5-hydroxymethylfurfural from glucose
The production of 5-hydroxymethylfurfural (5-HMF) from saccharide conversion has fallen short of industrial expectations due to low reaction efficiency and high costs. Herein, we fabricated a series of LaPO4 catalysts with controlled crystal structures and tuned acidity via a facile hydrothermal method. The hexagonal LaPO4-120 catalyst exhibited a high glucose conversion of 99.9% and a remarkable 5-HMF yield of 90.1% at 150 °C for 60 min. Additionally, the hexagonal LaPO4-120 catalyst showed promising activities in converting various saccharides including fructose, glucose, cellobiose, sucrose, and inulin. A series of characterization studies and DFT calculations revealed that the hexagonal LaPO4-120 catalyst possessed low-coordination La sites and abundant zeolitic water stored in the open and oxygen-lined channels, which provided Lewis acid sites and a dynamically local Brønsted acid environment to respectively facilitate glucose isomerization and the subsequent dehydration. A plausible reaction mechanism involving a synergetic proton transfer via hydrogen bonding is proposed. This study offers insights for the design of low-cost and heterogeneous catalysts with finely tuned Lewis and Brønsted acid sites for 5-HMF production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


