通过糖基的区域特异性和立体特异性氨基硒化,无金属合成多种2-脱氧β- n -糖苷
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
2-脱氧-β- n -糖苷及其类似物是天然产物和药物的关键成分。然而,由于缺乏邻基辅助,它们的立体选择性合成仍然是一个重大挑战。在此,我们提出了一种高效且无金属的方法,通过苯基碘双氟乙酸酯(PIFA)促进糖基的区域和立体特异性氨基硒化,利用易于获取的供体和n -杂环合成结构多样的2-苯基硒-β- n -糖苷。该方法具有反应条件温和、原子经济性高、易于扩展和底物范围广(76例)等特点,可通过快速脱硒合成立体定向的2-脱氧-β- n -糖苷。合成的实用性是通过后期药学相关分子的功能化和各种下游合成转化来证明的。实验和计算研究表明了一种自由基-极性交叉途径,并揭示了β-立体选择性的潜在原因。总的来说,本工作为快速获取有价值的2-脱氧-β- n-糖苷提供了一种有效的方法,并将促进其深入的生物学评价。本文章由计算机程序翻译,如有差异,请以英文原文为准。
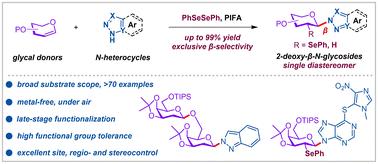
Metal-free synthesis of diverse 2-deoxy-β-N-glycosides through regio- and stereospecific aminoselenenylation of glycals
2-Deoxy-β-N-glycosides and their analogues are key components of natural products and pharmaceuticals. However, their stereoselective synthesis remains a significant challenge due to lack of neighboring group assistance. Herein, we present an efficient and metal-free method for synthesizing structurally diverse 2-phenylseleno-β-N-glycosides via phenyliodine bistrifluoroacetate (PIFA)-promoted regio- and stereospecific aminoselenenylation of glycals utilizing readily accessible donors and N-heterocycles. This approach features mild reaction conditions, high atom economy, easy scalability and broad substrate scope (76 examples), enabling stereospecific synthesis of 2-deoxy-β-N-glycosides via a facile deselenization. The synthetic utility is demonstrated by late-stage functionalization of pharmaceutically relevant molecules and various downstream synthetic transformations. Experimental and computational studies suggest a radical-polar crossover pathway and reveal the underlying reasons for β-stereoselectivity. Overall, the present work provides an efficient approach for rapid access to valuable 2-deoxy-β-N-glycosides, and will facilitate their in-depth biological evaluations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


