通过硫化双金属氢氧化物来控制边缘活性位点,促进析氧反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
含硫废水可以作为合成过渡金属硫化物(TMS)电催化剂的宝贵资源,以解决水裂解过程中析氧反应(OER)动力学缓慢的问题,这是影响环境和能源进步的重要问题。然而,tms受到内在的限制,特别是普遍存在的催化惰性基面阻碍了它们的功效。为了解决这一问题,本文采用含硫废水合成的乙基黄药钠在泡沫镍(NF)上构建了CoNi - s /CoNi(OH)2纳米片,对CoNi(OH)2进行了部分硫化,提高了OER性能。在CoNi(OH)2纳米片上原位形成的Co9S8-Ni3S2提供了高密度的边缘活性位点,补偿了惰性基面。由于S2−和O2−的桥接作用,电子重分布有利于Ni3+的形成,从而促进了CoNi - s /CoNi(OH)2向活性含s CoNiOOH的相变。密度泛函理论计算证实,将S加入到CoNi(OH)2中会导致丰富的边缘催化位点,并调节含氧中间体的吸附/解吸,显著降低OER过程中电位决定步骤的能量势垒。此外,S2−和O2−通过π给予Mn+,强化M - s (M = Co, Ni)键,提高了CoNi - s /CoNi(OH)2的稳定性。这些特性使得CoNi - s /CoNi(OH)2在20 mA cm−2时具有295 mV的低过电位,Tafel斜率为58 mV dec−1。这项工作提出了一种在清洁能源应用中利用含硫废水的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
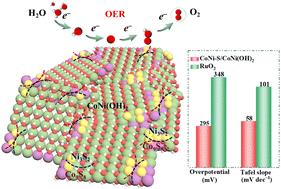
Controlling edge active sites by vulcanizing a bimetallic hydroxide for boosting the oxygen evolution reaction
Sulfur-laden wastewater can serve as a valuable resource for synthesizing transition-metal sulfide (TMS) electrocatalysts to address the sluggish kinetics of the oxygen evolution reaction (OER) in water splitting, which is a significant issue affecting environmental and energy advancements. However, TMSs suffer from intrinsic constraints, particularly a prevalence of catalytically inert basal planes that impede their efficacy. To solve this issue, herein, CoNi–S/CoNi(OH)2 nanosheets on nickel foam (NF) were constructed employing sodium ethyl xanthate (synthesized using sulfur-containing wastewater) to partially vulcanize CoNi(OH)2 for improving the OER performance. In situ formed Co9S8–Ni3S2 on CoNi(OH)2 nanosheets provide a high density of edge active sites, compensating for the inert basal planes. Due to the bridging effects of S2− and O2−, the electronic redistribution is conducive to forming Ni3+, thereby facilitating a phase transformation from CoNi–S/CoNi(OH)2 into active S-containing CoNiOOH. Density functional theory calculations verify that incorporating S into CoNi(OH)2 leads to rich edge catalytic sites and modulates the adsorption/desorption of oxygen-containing intermediates, significantly reducing the energy barrier of the potential-determining step during the OER process. Additionally, the stability of CoNi–S/CoNi(OH)2 was enhanced by the strengthened M–S (M = Co, Ni) bonds via π donation to Mn+ from S2− and O2−. All these features endow CoNi–S/CoNi(OH)2 with a low overpotential of 295 mV at 20 mA cm−2 with a Tafel slope of 58 mV dec−1. This work presents a strategy for utilizing sulfur-containing wastewater in clean energy applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


