Fe3S4/Ni3S2异质结构实现了乙二醇和碱性电解质高效电催化生产高附加值化学品和氢气
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电催化水裂解被认为是一种绿色、有前途的制氢方法。用热力学有利的乙二醇氧化反应(EGOR)取代缓慢的析氧反应(OER),实现了节能制氢和高价值甲酸盐生成。本文通过简单的一锅溶剂热策略,成功合成了自支撑Fe3S4/Ni3S2 (NiFeS)异质结电催化剂。在OER和EGOR过程中,由表面不均匀的熔融薄片覆盖的相互连接的纳米片最大限度地暴露了电活性位点。因此,电极只需要较低的OER过电压240 mV买得起100毫安的电流密度在1.0厘米−2 KOH电解质,并能稳定工作120 h。此外,只有1.37 V和流值需要达到100毫安的电流密度在1.0厘米−2 KOH乙二醇电解质+ 1.0米,和最高的法拉第效率(92.4%)和快速的生产率(0.652更易厘米−2 h−1)甲酸生产可以实现在一个1.50 V和流值的应用潜力。拉曼光谱结果表明,表面重构生成的金属氢氧化物(NiOOH和FeOOH)和金属硫化物分别是OER和EGOR的真正活性物质。HER(商用Pt/C电极)和EGOR (NiFeS电极)耦合电解系统在1.55 V下输出电流密度为100 mA cm - 2,比传统水电解系统低140 mV。本研究提出了一种合理的制备异质结催化剂的策略,用于碱性介质中节能制氢和增值制甲酸酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
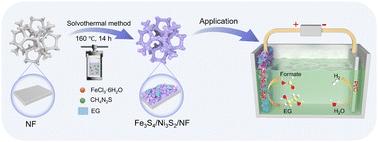
An Fe3S4/Ni3S2 heterostructure realizing highly efficient electrocatalysis of ethylene glycol and alkaline electrolyte to produce high value-added chemicals and hydrogen
Electrocatalytic water splitting is considered a green and promising strategy for hydrogen production. Replacing the sluggish oxygen evolution reaction (OER) with a thermodynamically favorable ethylene glycol oxidation reaction (EGOR) enables energy-saving hydrogen production coupled with high-value formate generation. Herein, a self-supported Fe3S4/Ni3S2 (NiFeS) heterojunction electrocatalyst was successfully synthesized through a facile one-pot solvothermal strategy. The interconnected nanosheets covered by fused flakes with uneven surfaces maximize the exposure of electroactive sites during the OER and the EGOR. Therefore, the electrode only requires a low OER overpotential of 240 mV to afford a current density of 100 mA cm−2 in 1.0 M KOH electrolyte, and can work stably for 120 h. Furthermore, only 1.37 V vs. RHE was required to achieve a current density of 100 mA cm−2 in 1.0 M KOH + 1.0 M ethylene glycol electrolyte, and the highest Faraday efficiency (92.4%) and rapid productivity (0.652 mmol cm−2 h−1) could be achieved for formate production at an applied potential of 1.50 V vs. RHE. The Raman spectroscopy indicated that the metal oxyhydroxides (NiOOH and FeOOH) generated by surface reconstruction and metal sulfides are the real active species of the OER and the EGOR, respectively. The coupled electrolysis system involving the HER (commercial Pt/C electrode) and the EGOR (NiFeS electrode) outputs a current density of 100 mA cm−2 at 1.55 V, which is 140 mV lower than that of the traditional water electrolysis system. This study puts forward a rational strategy for preparing heterojunction catalysts for energy-saving H2 and value-added formate production from alkaline media.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


