利用nhc稳定的各种金属和合金水溶性纳米颗粒探索5-羟甲基糠醛加氢途径
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
5-羟甲基糠醛(5-HMF)是一种关键的生物基平台分子,其催化价化是可持续化工生产的核心。在这项工作中,我们报道了用n -杂环碳烯(IMesPrSO3)配体稳定的水溶性纳米颗粒家族的5-羟甲基糠醛的水相加氢。采用BF-TEM、DLS、TGA、ICP-OES和广角x射线散射(WAXS)对分布函数(PDF)分析对纳米颗粒进行了全面表征。通过仅改变金属核心(Ru, Pd, Ir, RuIr2),并稍微改变反应条件,我们获得了各种高价值产品,包括2,5-二(羟甲基)呋喃(2,5- bhmmf), 2,5-二(羟甲基)四氢呋喃(2,5- bhmthf),氧化环戊酮和1-羟基己烷-2,5-二酮(HHD),所有这些产品都在环境友好的条件下(30-140°C, 5 bar H2,水中)。具体来说,钌基纳米颗粒在非常温和的条件下(30°C)对2,5- bhmf具有很高的选择性(90%),并且具有很好的可回收性。通过广泛的NMR波谱,包括1H、13C、COSY、HSQC和HMBC实验,对反应产物进行鉴定和定量。我们的研究结果表明,这些真正的胶体催化系统可以代表绿色和可持续生物质转化的可调和强大的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
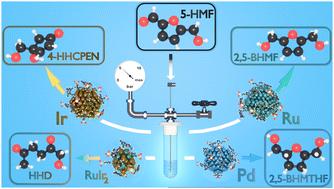
Exploring 5-hydroxymethylfurfural hydrogenation pathways using NHC-stabilized water-soluble nanoparticles of various metals and alloys†
The catalytic valorization of 5-hydroxymethylfurfural (5-HMF), a key bio-based platform molecule, is central for sustainable chemical production. In this work, we report the aqueous-phase hydrogenation of 5-HMF using a family of water-soluble nanoparticles stabilized by an N-heterocyclic carbene (IMesPrSO3) ligand. The nanoparticles were comprehensively characterized by BF-TEM, DLS, TGA, ICP-OES, and pair distribution function (PDF) analysis from wide-angle X-ray scattering (WAXS). By only varying the metal core (Ru, Pd, Ir, RuIr2), and slightly modifying the reaction conditions, we accessed a diverse array of high-value products, including 2,5-bis(hydroxymethyl)furan (2,5-BHMF), 2,5-bis(hydroxymethyl)tetrahydrofuran (2,5-BHMTHF), oxidized cyclopentenones, and 1-hydroxyhexane-2,5-dione (HHD), all under environmentally friendly conditions (30–140 °C, 5 bar H2, in water). Specifically, the Ru-based nanoparticles showed high selectivity to 2,5-BHMF (90%) at very mild conditions (30 °C), along with a promising recyclability. Reaction products were identified and quantified through extensive NMR spectroscopy, including 1H, 13C, COSY, HSQC, and HMBC experiments. Our findings demonstrate that these truly colloidal catalytic systems can represent a tuneable and robust platform for green and sustainable biomass transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


