针对人载脂蛋白100致病表位的三体免疫分析方法的开发,用于动脉粥样硬化的诊断和监测
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
心血管疾病是全球头号致命疾病,动脉粥样硬化是其病理基础。然而,动脉粥样硬化的发展是慢性和无症状的,对其早期发现和及时治疗提出了重大挑战。最近,低密度脂载脂蛋白B-100 (ApoB100)中的3136-3155氨基酸序列p210被发现与动脉粥样硬化的严重程度和心血管事件的风险相关。为了建立一种精确、灵敏的动脉粥样硬化早期诊断免疫检测方法,本研究构建了3个针对人ApoB100 p210肽的单链片段变量(single chain fragment variable, scFv)噬菌体文库,获得了3个高亲和力的scFv,并将其基因工程构建成一个三体,显著增强了其对致病p210肽的功能亲和力和抗原捕获能力。此外,开发了一种快速简便的方法,用于临床环境中疾病进展的早期监测。含该三体夹心ELISA的线性范围为36 ~ 576 ng/mL,检出限为28.5 ng/mL。平均回收率为88.4±3.0%,平均回收率为85.5±5.2%。该方法对病原菌表位具有高度的敏感性和特异性,且与血清中其他抗原无交叉反应。重要的是,由于与三聚体scFv形成的针对相同表位的三聚体抗体的亲和力比具有单体scFv的正常抗体高2.5倍,因此其衍生的夹心ELISA可以在极低水平下测量致病表位的数量,从而能够在动脉粥样硬化早期患者中检测出与疾病严重程度相对应的信号。总的来说,噬菌体文库衍生的三体体成功地转化为一种对致病表位具有更高亲和力的快速免疫测定方法,不仅可以早期检测,还可以实时监测疾病进展,以最大限度地降低动脉粥样硬化性心血管疾病的风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
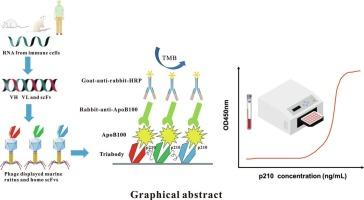
Development of a triabody-based immunoassay against a pathogenic epitope in human ApoB100 for the diagnosis and monitoring of atherosclerosis
Cardiovascular diseases are the number one deadly diseases worldwide, with atherosclerosis being their pathological basis. However, the development of atherosclerosis is chronic and asymtomatic, posing significant challenges for its early detection and timely treatment. Recently, a 3136–3155 amino acid sequence, p210, in the apolipoprotein B-100 (ApoB100) of low density lipid was found to be associated with the severity of atherosclerosis and the risk of cardiovascular events. To develop a precise and sensitive immunoassay for the early diagnosis of atherosclerosis, three phage libraries displaying single chain fragment variable (scFv) against the p210 peptide in human ApoB100 were constructed in the present study, and three high affinity scFvs obtained, which were then genetically engineered to constitute a triabody that significantly enhanced its functional affinity and antigen-capturing capability towards the pathogenic p210 peptides. Furthermore, a rapid and simple method was developed with the triabody for early monitoring of the disease progression in clinical settings. The linear range of the sandwich ELISA containing the triabody was 36–576 ng/mL, with a limit of detection of 28.5 ng/mL. In addition, the average recovery of intra- and inter-assay were 88.4 ± 3.0 % and 85.5 ± 5.2 %, respectively. The developed assay was highly sensitive and specific for the pathogenic epitope, and no cross-reactivity with other antigens in serum observed. Importantly, since the triabody developed with trimeric scFvs against the same epitope has 2.5 times higher affinity than that of normal antibody with monomeric scFv, its derived sandwich ELISA could measure the amounts of pathogenic epitopes at very low levels, thereby capable of detecting signals corresponding to the severity of the disease in patients at early stage of atherosclerosis. Collectively, the phage library-derived triabody were successfully translated into a quick immunoassay with higher affinity against a pathogenic epitope that allows not only early detection but also real-time monitoring of the disease progression to minimize the risk of atherosclerotic cardiovascular disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


