具有独特结构和优异催化活性的新型离子液体的设计与合成
IF 7.5
3区 环境科学与生态学
Q2 ENERGY & FUELS
引用次数: 0
摘要
采用一步法合成了一系列具有近中性、中等亲核性和两性离子结构的新型离子液体。值得注意的是,这些il可以通过碳酸酯、羧酸酯和草酸酯活化1-甲基咪唑成功合成。采用傅里叶变换红外光谱、1H/13C、核磁共振光谱、热重质谱和Hammett指示剂对合成的ILs的结构和性质进行定性和定量分析。提出了1-甲基咪唑的活化机理,并计算了其负电荷密度。以MI-EC为例,优化了合成il的最佳条件,在85℃下反应18 h,通过密度泛函理论计算确定了合成途径。其中,MI-EC在酯交换反应中表现出优异的催化活性,相应的碳酸乙酯(EC)转化率、碳酸二甲酯(DMC)产率和周转频率(TOF)分别达到50.4%、30.5%和127.8 h−1,催化反应时间仅为30 min。进一步探讨了MI-EC催化酯交换反应的机理。6次重复使用后,MI-EC的催化活性和结构保持不变,表明其具有较好的稳定性。此外,MI-EC显示出广泛的底物普遍性,如碳酸盐,草酸盐和乙酸酯。因此,本研究不仅为设计和合成il提供了理论和实践支持基础,而且为制备碱性催化剂提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
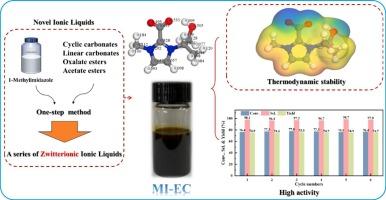
Design and synthesis of novel ionic liquids with unique structures and excellent catalytic activity for transesterification
A series of novel ionic liquids (ILs) with near-neutrality, moderate nucleophilicity, and zwitterionic structure were synthesized using a one-step method. Notably, these ILs could be successfully synthesized by activating 1-methylimidazole with carbonate, carboxylic, and oxalate esters. The structures and properties of the synthesized ILs were qualitatively and quantitatively analyzed using Fourier-transform infrared spectroscopy, 1H/13C, nuclear magnetic resonance spectroscopy, thermogravimetry–mass spectrometry, and Hammett indicator. A mechanism was proposed for activating 1-methylimidazole, and the negative charge densities of the ILs were calculated. Considering MI-EC as an example, the best conditions for the synthesis of ILs were optimized reaction at 85 °C for 18 h, and the synthesis pathway was determined through density functional theory calculations. Herein, MI-EC exhibited excellent catalytic activity for transesterification reactions, and the corresponding ethylene carbonate (EC) conversion, dimethyl carbonate (DMC) yield, and turnover frequency (TOF) reached 50.4 %, 30.5 %, and 127.8 h−1, respectively, with a catalytic reaction of only 30 min. Furthermore, the mechanism underlying the transesterification reaction catalyzed by MI-EC was investigated. The catalytic activity and structure of MI-EC remained unchanged after six reuses, demonstrating its better stability. In addition, MI-EC displayed a wide range of substrate universality, such as carbonates, oxalates, and acetic esters. Thus, this study not only provides a theoretical and practical support foundation for designing and synthesizing ILs, but also provides a new perspective for preparing alkaline catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon Resources Conversion
Materials Science-Materials Science (miscellaneous)
CiteScore
9.90
自引率
11.70%
发文量
36
审稿时长
10 weeks
期刊介绍:
Carbon Resources Conversion (CRC) publishes fundamental studies and industrial developments regarding relevant technologies aiming for the clean, efficient, value-added, and low-carbon utilization of carbon-containing resources as fuel for energy and as feedstock for materials or chemicals from, for example, fossil fuels, biomass, syngas, CO2, hydrocarbons, and organic wastes via physical, thermal, chemical, biological, and other technical methods. CRC also publishes scientific and engineering studies on resource characterization and pretreatment, carbon material innovation and production, clean technologies related to carbon resource conversion and utilization, and various process-supporting technologies, including on-line or off-line measurement and monitoring, modeling, simulations focused on safe and efficient process operation and control, and process and equipment optimization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


