腺苷2A受体平衡信号激活的结构基础依赖于变构介导的结构动力学
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
腺苷2A受体(A2AAR)平衡或偏置的G蛋白和阻滞蛋白跨膜信号传导与配体诱导的跨膜结构域(TMD)胞内半区变构触发的结构动力学变化有关。在TMD核心的遗传引入的meta-三氟甲基- l-苯丙氨酸(mtfF)探针网络的19f -核磁共振(NMR)揭示了从细胞外正位药物结合位点到G蛋白和细胞内表面阻滞蛋白接触的信号相关结构重排。信号传递的这种结构基础的关键因素是TMD细胞内一半结构秩序的动态丢失,这表现在先前由x射线晶体学确定的分子结构中的局部多态性和相关速率过程中。G蛋白偶联受体(GPCR)激活的结构基础可视化为优化药物设计中的偏置信号提供了另一种范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
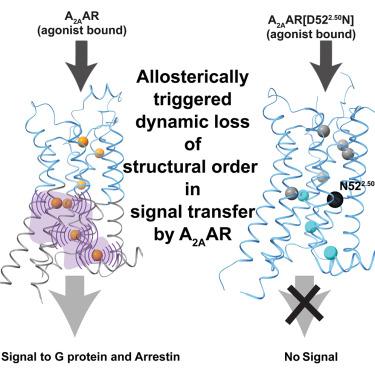
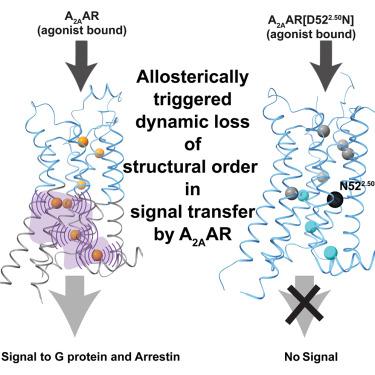
Structural basis of adenosine 2A receptor-balanced signaling activation relies on allosterically mediated structural dynamics
Balanced or biased G protein and arrestin transmembrane signaling by the adenosine 2A receptor (A2AAR) is related to ligand-induced allosterically triggered variation of structural dynamics in the intracellular half of the transmembrane domain (TMD). 19F-nuclear magnetic resonance (NMR) of a network of genetically introduced meta-trifluoromethyl-L-phenylalanine (mtfF) probes in the core of the TMD revealed signaling-related structure rearrangements leading from the extracellular orthosteric drug-binding site to the G protein and arrestin contacts on the intracellular surface. The key element in this structural basis of signal transfer is dynamic loss of structural order in the intracellular half of the TMD, as manifested by local polymorphisms and associated rate processes within the molecular architecture determined previously by X-ray crystallography. This visualization of the structural basis of G protein-coupled receptor (GPCR) activation presents an alternative paradigm for optimizing biased signaling in drug design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


