小分子fgfr靶向药物化学:自2020年以来的进展和未来展望
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
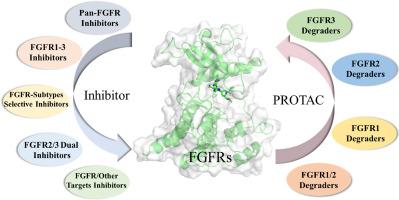
FGFR改变,包括融合、扩增、重排和突变,在许多疾病和癌症中作为致病驱动因素或旁路机制存在。因此,fgfr代表了重要的治疗靶点,特别是在肿瘤学领域。各种药物,特别是选择性FGFR抑制剂,在肿瘤疾病中显示出良好的治疗潜力。然而,与FGFR抑制剂相关的脱靶毒性(如高磷血症)和获得性耐药往往导致疾病进展和患者的不利结果,限制了临床应用。这推动了下一代FGFR疗法的发展,特别是增强同种异构体的选择性和克服耐药性突变。本文综述了FGFR蛋白的结构、生物学功能和疾病相关性,同时重点介绍了FGFR抑制剂和降解剂的进展(2020年至今),包括设计策略、SARs、结合模式和生物学评价。此外,本文还讨论了亚型选择性和耐药突变的独特机制,为开发改进的fgfr靶向药物提供了战略见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Small-molecule FGFR-targeted medicinal chemistry: Advances since 2020 and future perspectives
FGFR alterations, including fusions, amplifications, rearrangements, and mutations, exist as pathogenic drivers or bypass mechanisms in numerous diseases and cancers. Thus, FGFRs represent crucial therapeutic targets, particularly in oncology. Various agents, especially selective FGFR inhibitors, have shown promising therapeutic potential in oncological diseases. However, off-target toxicities (e.g., hyperphosphatemia) and acquired drug resistance associated with FGFR inhibitors often result in disease progression and unfavorable outcomes for patients, constraining clinical utility. This drives next-generation FGFR therapeutics development, particularly enhancing isoform selectivity and overcoming resistance mutations. This review summarizes FGFR protein architecture, biological functions, and disease associations, while highlighting advances (2020–present) in FGFR inhibitors and degraders, including design strategies, SARs, binding modes, and biological evaluation. Additionally, the unique mechanisms underlying subtype selectivity and resistance to drug-resistant mutations are discussed, providing strategic insights for developing improved FGFR-targeted agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


