B细胞来源的LTα3和LTα1β2在tnf介导的回肠炎中的不同作用
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
在TNFΔARE+/−小鼠中模拟克罗恩病病理,这些小鼠过度产生肿瘤坏死因子(TNF),通过TNF受体驱动疾病。TNF受体的另一种配体,可溶性LTα3,是由B细胞产生的,但很少受到关注,因为LTα也与LTβ一起产生膜系住的LTαβ2,促进三级淋巴组织-克罗恩病的另一个特征。我们假设B细胞来源的LTαβ2会严重影响TNFΔARE+/−小鼠的回肠炎。然而,尽管删除B细胞中的LTβ对于三级淋巴组织是必需的,但对疾病病理的影响很小。相比之下,B细胞源性LTα的缺失增加了肠道通透性,缩小了IgA+回肠浆细胞池,细胞因子升高,并促使体重减轻,包括肌肉质量的减少——这是克罗恩病的系统性特征。LTα3中和抗体强烈增强了TNF的恶病质样作用。因此,B细胞产生的LTαβ2和LTα3在回肠炎中具有不同的作用,其中LTα3通过平衡TNF发挥意想不到的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
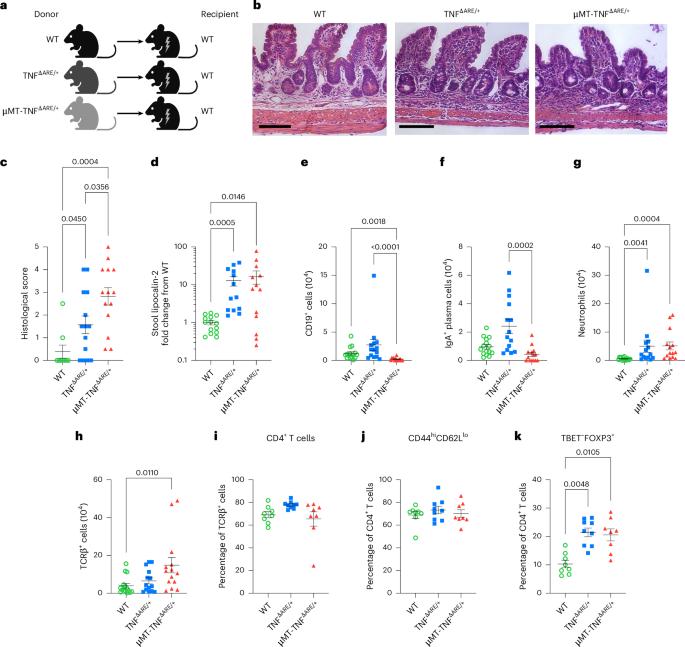
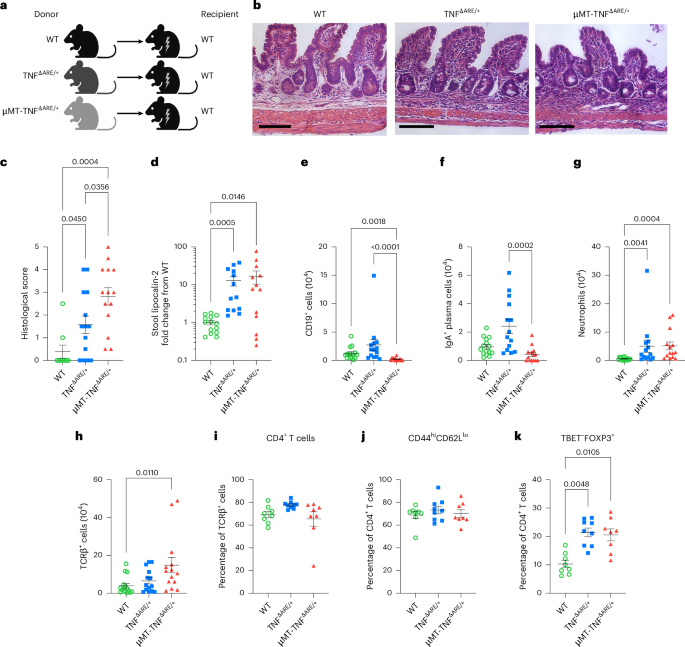
Distinct roles for B cell-derived LTα3 and LTα1β2 in TNF-mediated ileitis
Crohn’s disease pathology is modeled in TNFΔARE+/− mice that overproduce tumor necrosis factor (TNF) to drive disease through TNF receptors. An alternative ligand for TNF receptors, soluble LTα3, is produced by B cells, but has received scarce attention because LTα also partners with LTβ to generate membrane-tethered LTαβ2 that promotes tertiary lymphoid tissue—another feature of Crohn’s disease. We hypothesized that B cell-derived LTαβ2 would critically affect ileitis in TNFΔARE+/− mice. However, whereas deleting LTβ in B cells was essential for tertiary lymphoid tissue, disease pathology was minimally affected. By contrast, loss of B cell-derived LTα increased intestinal permeability, shrunk the pool of IgA+ ileal plasma cells, elevated cytokines and prompted weight loss, including loss of muscle mass—a systemic feature of Crohn’s disease. Neutralizing antibodies to LTα3 strongly augmented the cachexic-like effects of TNF. Thus, B cell-produced LTαβ2 and LTα3 have distinct roles in ileitis, with the role of LTα3 unexpectedly protective through counterbalancing TNF. Erlich et al. show that soluble LTα and membrane-bound LTα1β2 lymphotoxins expressed by B cells play distinct roles to attenuate the pathology observed in ileitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


