实现单选择性钯(II)催化芳烃与蛋白质配体的碳氢活化
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
当多个相同的碳氢键共存时,在碳氢活化反应中实现单选择性是相当大的挑战。尽管近年来在位点选择性和对映选择性C-H活化方面进展迅速,但大量的C-H活化反应仍然存在较差的单选择性。在这里,我们报告了使用商业酶作为钯催化剂的配体,在芳烃的邻位和间碳氢活化中增强了反应活性和极高的单选择性(高达99%),而芳烃最初使用双功能单n保护氨基酸配体,但单选择性较差。值得注意的是,pd -酶配合物被确定为活性催化剂。酶的机制研究和结构分析表明,酶的一级结构、序列长度和疏水侧链氨基酸的百分比是实现单选择性的关键。利用这些发现,我们进一步开发了一种含有甘氨酸的寡肽,能够实现类似的高单选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

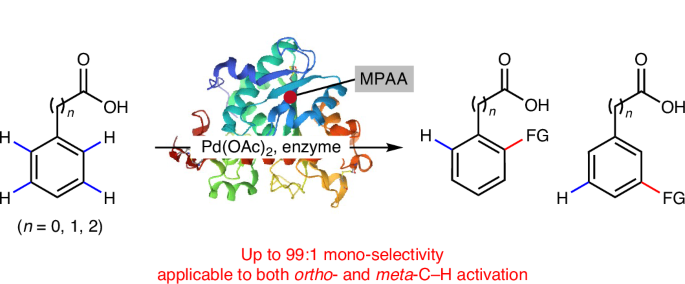
Achieving mono-selective palladium(II)-catalysed C–H activation of arenes with protein ligands
Achieving mono-selectivity in C–H activation reactions is a considerable challenge when multiple identical C–H bonds coexist. Despite recent rapid advances in site-selective and enantioselective C–H activation, a large number of C–H activation reactions still suffer from poor mono-selectivity. Here we report the use of commercial enzymes as ligands for palladium catalysts, enabling enhanced reactivity and exceptionally high mono-selectivity (up to 99%) in both ortho- and meta-C–H activation of arenes, which originally used bifunctional mono-N-protected amino acid ligands but with poor mono-selectivity. Notably, the Pd–enzyme complex was identified as the active catalyst species. Mechanistic investigations and structural analyses of the enzymes suggest that the enzyme primary structure, the sequence length and the percentage of amino acids with hydrophobic side chains are critical for achieving mono-selectivity. By leveraging these findings, we further developed a glycine-containing oligopeptide capable of achieving similarly high mono-selectivity. Molecular organometallic catalysts typically struggle to activate only one of two identical C–H bonds in arenes for mono-selective C–H activation. Now mono-selectivity has been achieved for Pd(II)-catalysed ortho- or meta-C–H activations using commercial proteins or designed peptides as ligands.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


