亚氨基膦胺基乙烯能定量地将CO₂转化为异氰酸酯
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亚磷酰胺负载的硅氨基丁烯通过氨基丁烯中间体活化CO₂生成硅氧硅氧烷和异氰酸酯。DFT计算支持涉及亲核攻击和硅基迁移的两步重排,提供了一个罕见的通过异氰酸酯形成主基CO₂增值的例子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
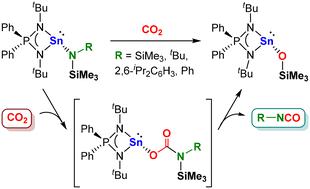
Iminophosphonamido stannylenes enable quantitative CO2 conversion to isocyanates
Iminophosphonamido-supported silylaminostannylenes activate CO2 to afford siloxystannylene and isocyanates via carbamatostannylene intermediates. DFT calculations support a two-step rearrangement involving nucleophilic attack and silyl migration, offering a rare example of a main-group CO2 valorisation through isocyanate formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


