一种新的动态通量平衡分析,用于模拟pH和温度变化的CHO细胞补料批培养。
IF 3.9
2区 生物学
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
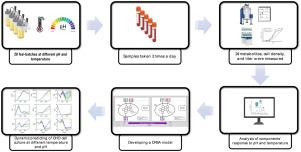
虽然动态通量平衡分析为模拟代谢行为提供了一个强大的框架,但将影响单克隆抗体产生的操作条件(如pH和温度)纳入其中仍然具有挑战性。本研究提出了一个先进的dFBA模型,该模型集成了作为pH和温度函数的动力学约束,以预测不同操作条件下CHO细胞的代谢。该模型通过在Ambr®250生物反应器中进行的20个进料批实验数据进行了验证。为了减轻过度参数化,采用贝叶斯信息准则的双层优化方法系统地识别最有效的动力学约束。这种优化减少了参数的数量(从253个减少到205个),同时将训练数据集的预测精度提高了8.3%,验证数据集的预测精度提高了2.68%。结果表明,该模型具有预测细胞生长、滴度和捕获代谢变化的能力,包括葡萄糖、乳酸、氨代谢和氨基酸利用,在不同温度和pH条件下具有很高的预测精度(细胞生长和滴度的平均R2≥0.97,其他代谢物的平均R2≥0.85)。这个优化的dFBA框架为研究CHO细胞代谢的基于模型的优化提供了一个强大的工具,确定了平衡生长和生产力的最佳操作条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A novel dynamic flux balance analysis for modeling CHO cell fed-batch cultures with pH and temperature shifts
While Dynamic Flux Balance Analysis provides a powerful framework for simulating metabolic behavior, incorporating operating conditions such as pH and temperature, which profoundly impact monoclonal antibodies production, remains challenging. This study presents an advanced dFBA model that integrates kinetic constraints formulated as functions of pH and temperature to predict CHO cell metabolism under varying operational conditions. The model was validated against data from 20 fed-batch experiments conducted in Ambr®250 bioreactors. To mitigate overparameterization, a bi-level optimization approach utilizing the Bayesian Information Criterion was employed to systematically identify the most effective kinetic constraints. This optimization reduced the number of parameters (from 253 to 205) while improving predictive accuracy by up to 8.3% for training and 2.68% for validation datasets. The results highlight the model’s ability to predict cell growth, titer, and also capture metabolic shifts, including glucose, lactate, and ammonia metabolism and amino acid utilization, across different temperature and pH conditions with high predictive precision (average for cell growth and titer and average for other metabolites). This optimized dFBA framework offers a robust tool for studying model-based optimization for CHO cell metabolism, identifying optimal operating conditions to balance growth and productivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of biotechnology
工程技术-生物工程与应用微生物
CiteScore
8.90
自引率
2.40%
发文量
190
审稿时长
45 days
期刊介绍:
The Journal of Biotechnology has an open access mirror journal, the Journal of Biotechnology: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The Journal provides a medium for the rapid publication of both full-length articles and short communications on novel and innovative aspects of biotechnology. The Journal will accept papers ranging from genetic or molecular biological positions to those covering biochemical, chemical or bioprocess engineering aspects as well as computer application of new software concepts, provided that in each case the material is directly relevant to biotechnological systems. Papers presenting information of a multidisciplinary nature that would not be suitable for publication in a journal devoted to a single discipline, are particularly welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


