双作用纳米疗法:替莫唑胺负载,抗pd - l1 scfv功能化脂质纳米载体靶向胶质母细胞瘤治疗。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
胶质母细胞瘤(GBM)是一种高度恶性的脑肿瘤,治疗方案有限,预后差。GBM对包括替莫唑胺(TMZ)、放疗和免疫治疗在内的常规治疗具有耐药性,部分原因是由于程序性死亡配体1 (PD-L1)过表达等免疫抑制机制。为了解决这些挑战,我们开发了装载tmz的纳米结构脂质载体(NLCs),结合抗pd - l1单链可变片段(scFv),用于双重化学免疫治疗。抗pd - l1 scFv能够主动靶向表达pd - l1的肿瘤细胞,同时减轻免疫逃避,NLCs有效地穿过血脑屏障(BBB),将TMZ传递到肿瘤部位。体外研究证实了纳米颗粒被GL261胶质瘤细胞内化,并且结合的scFv特异性结合PD-L1。使用GL261/C57BL/6小鼠模型的体内研究表明,与其他治疗组不同,TMZ-NLCs结合抗pd - l1 scFv (TMZ-NP-scFv)在第三周稳定了肿瘤生长。虽然与对照组相比,所有治疗组(TMZ溶液、TMZ- np和单独抗pd - l1 scFv)均显著改善了生存,但TMZ- np -scFv组表现出最明显的生存益处。这些结果强调了装载tmz的pd - l1靶向NLCs作为一种协同策略的潜力,通过联合化疗和免疫检查点阻断来增强GBM的治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
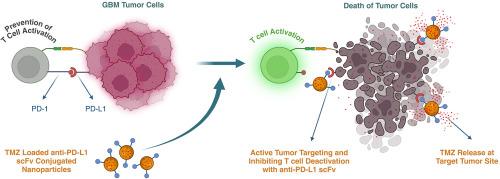
Dual-action nanotherapy: Temozolomide-loaded, anti-PD-L1 scFv-functionalized lipid nanocarriers for targeted glioblastoma therapy
Glioblastoma (GBM) is a highly malignant brain tumor with limited treatment options and poor prognosis.
GBM exhibits resistance to conventional therapies, including temozolomide (TMZ), radiotherapy, and immunotherapy, partly due to immunosuppressive mechanisms such as programmed death-ligand 1 (PD-L1) overexpression. To address these challenges, we developed TMZ-loaded nanostructured lipid carriers (NLCs) conjugated with anti-PD-L1 single-chain variable fragments (scFv) for dual chemo-immunotherapy.
The anti-PD-L1 scFv enabled active targeting of PD-L1-expressing tumor cells while mitigating immune evasion, and the NLCs efficiently crossed the blood-brain barrier (BBB), delivering TMZ to the tumor site. In vitro studies confirmed nanoparticle internalization by GL261 glioma cells and specific binding of the conjugated scFv to PD-L1. In vivo studies using the GL261/C57BL/6 mouse model demonstrated that TMZ-NLCs conjugated with anti-PD-L1 scFv (TMZ-NP-scFv) stabilized tumor growth by the third week, unlike other treatment groups. While all therapeutic groups (TMZ solution, TMZ-NP, and anti-PD-L1 scFv alone) significantly improved survival compared to controls, the TMZ-NP-scFv group exhibited the most pronounced survival benefit.
These results highlight the potential of TMZ-loaded, PD-L1-targeted NLCs as a synergistic strategy to enhance GBM treatment by combining chemotherapy and immune checkpoint blockade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


