苯通过诱导铁下垂和下调GPX4诱导骨髓发育不良和血液毒性。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:苯是一种普遍存在的工业化学品,是一种公认的环境毒素,与血液系统疾病有关,如骨髓增生异常综合征(MDS)和急性髓性白血病(AML),其特征是造血功能受损和骨髓衰竭。本研究探讨了铁坏死(一种铁依赖性细胞死亡形式)在苯诱导的血液毒性中的作用,重点研究了谷胱甘肽过氧化物酶4 (GPX4)的抑制作用,GPX4是铁坏死的关键调节因子。材料与方法:雄性C57BL/6小鼠以不同剂量的苯暴露6周。采用生化分析、PCR和Western blotting方法评估骨髓和CD34+造血干细胞中的铁凋亡标志物、氧化应激水平和GPX4表达。通过crispr - cas9介导的GPX4敲除和铁下沉诱导剂erastin诱导铁下沉。并评价了铁下垂抑制剂(DFO和Fer-1)的保护作用。结果:苯暴露导致红细胞和白细胞计数、血红蛋白水平和骨髓细胞数量的剂量依赖性减少。它还会升高氧化应激标志物,破坏铁代谢,抑制GPX4的表达。GPX4敲除和erastin处理模拟苯诱导的效应,导致细胞活力下降,集落形成受损和脂质过氧化积累。施用铁下垂抑制剂逆转了这些作用,恢复细胞功能,减少活性氧(ROS),并防止氧化损伤。GPX4过表达进一步保护了苯诱导的铁下垂和造血毒性。结论:氧化应激、铁代谢破坏和GPX4抑制介导的铁下沉在苯诱导的血液毒性中起核心作用。下垂铁抑制剂显示出潜在的治疗药物减轻苯诱导的血液学损伤,为环境毒素引起的疾病提供新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
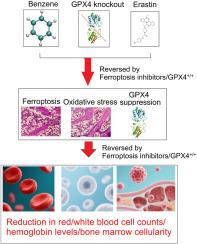
Benzene induces myelodysplasia and hematotoxicity via ferroptosis induction and downregulation of GPX4
Background
Benzene, a ubiquitous industrial chemical, is a well-established environmental toxin associated with hematological disorders such as myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), which are characterized by impaired hematopoiesis and bone marrow failure. This study investigates the role of ferroptosis, an iron-dependent form of cell death, in benzene-induced hematotoxicity, focusing on the repression of glutathione peroxidase 4 (GPX4), a critical regulator of ferroptosis.
Materials and methods
Male C57BL/6 mice were exposed to benzene at various doses over six weeks. Ferroptosis markers, oxidative stress levels, and GPX4 expression were assessed in bone marrow and CD34+ hematopoietic stem cells using biochemical assays, PCR, and Western blotting. Ferroptosis was induced via CRISPR-Cas9-mediated GPX4 knockout and the ferroptosis inducer erastin. The protective effects of ferroptosis inhibitors (DFO and Fer-1) were also evaluated.
Results
Benzene exposure led to a dose-dependent reduction in red and white blood cell counts, hemoglobin levels, and bone marrow cellularity. It also elevated oxidative stress markers, disrupted iron metabolism, and suppressed GPX4 expression. GPX4 knockout and erastin treatment mimicked benzene-induced effects, causing decreased cell viability, impaired colony formation, and lipid peroxidation accumulation. Administration of ferroptosis inhibitors reversed these effects, restoring cellular function, reducing reactive oxygen species (ROS), and preventing oxidative damage. GPX4 overexpression further protected against benzene-induced ferroptosis and hematopoietic toxicity.
Conclusion
Ferroptosis plays a central role in benzene-induced hematotoxicity, mediated by oxidative stress, disruption of iron metabolism, and suppression of GPX4. Ferroptosis inhibitors show potential as therapeutic agents for mitigating benzene-induced hematological damage, offering novel therapeutic strategies for disorders induced by environmental toxins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


