应用基于sigma的质量控制规则提高内部质量控制的效率
IF 1.3
Q3 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
摘要
背景:通过质量控制(QC)确保医学实验室的稳定性至关重要,需要适当的规则来防止虚假警报和识别错误。本研究展示了如何引入新的质量控制规则,以配合个人总允许误差(TEa)影响实验室效率和错误检测。方法对26项生化指标应用新内控规则前后的性能变化进行研究。前期采用统一的QC规则(1-3s、2-2s、2/3-2s、R-4s、4-1s和12-x),后期采用由Westgard Adviser (Bio-Rad Inc., USA)选择的新QC规则。与同行组相比,使用TEa和IQC数据的精度和偏倚来计算Sigma指标。通过比较QC-repeat率、周转时间(TAT)和熟练程度测试(PT)结果来评估效率。结果在前阶段,由于违规导致的qc -repeat平均为5.6%,在后阶段下降到2.5%。其结果是,在高峰时间,不符合tat的比率从29.4%下降到15.2%。在前期,271例患者中有67例超过PT的2标准差指数(SDI),在后期减少到24例。超过3sdi的病例在后期从27例明显减少到4例。在内部质量控制过程中引入基于西格玛的规则,在保持质量的同时,通过减少QC-repeat、重新校准和TAT,提高了实验室效率,展示了效率和分析性能之间的宝贵平衡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Application of sigma-based quality control rules for the efficiency of internal quality control
Background
Ensuring stability in medical laboratories through quality control (QC) is crucial and requires fitted rules to prevent false alerts and identify errors. This study demonstrates how the introduction of new QC rules to align with individual total allowable error (TEa) affects laboratory efficiency and error detection.
Methods
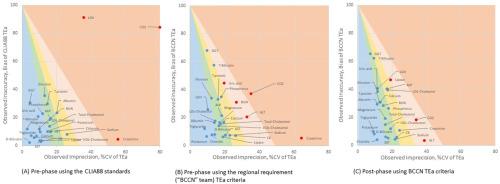
Changes in the performance of 26 biochemical tests before and after applying new internal quality control (IQC) rules were studied. Pre-Phase utilized uniform QC rules (1–3s, 2-2s, 2/3-2s, R-4s, 4-1s, and 12-x) while Post-Phase adopted new QC rules selected using Westgard Adviser (Bio-Rad Inc., USA). Sigma metrics were calculated using TEa and precision and bias from IQC data, compared to the peer group. Efficiency was assessed by comparing QC-repeat rates, turnaround times (TAT), and proficiency test (PT) results.
Results
QC-repeats due to violations averaged 5.6 % in the Pre-Phase and decreased to 2.5 % in the Post-Phase. As a result, the rate of out-of-TAT in peak-time decreased from 29.4 % to 15.2 %. In Pre-Phase, 67 of 271 cases exceeded the 2 standard deviation index (SDI) in the PT, which was reduced to 24 cases in Post-Phase. Cases exceeding the 3 SDI significantly decreased from 27 to 4 in the Post-Phase.
Conclusion
The introduction of sigma-based rules in the internal quality control process improved laboratory efficiency by reducing QC-repeat, recalibration, and TAT while maintaining quality, demonstrating a valuable balance between efficiency and analytical performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Practical Laboratory Medicine
Health Professions-Radiological and Ultrasound Technology
CiteScore
3.50
自引率
0.00%
发文量
40
审稿时长
7 weeks
期刊介绍:
Practical Laboratory Medicine is a high-quality, peer-reviewed, international open-access journal publishing original research, new methods and critical evaluations, case reports and short papers in the fields of clinical chemistry and laboratory medicine. The objective of the journal is to provide practical information of immediate relevance to workers in clinical laboratories. The primary scope of the journal covers clinical chemistry, hematology, molecular biology and genetics relevant to laboratory medicine, microbiology, immunology, therapeutic drug monitoring and toxicology, laboratory management and informatics. We welcome papers which describe critical evaluations of biomarkers and their role in the diagnosis and treatment of clinically significant disease, validation of commercial and in-house IVD methods, method comparisons, interference reports, the development of new reagents and reference materials, reference range studies and regulatory compliance reports. Manuscripts describing the development of new methods applicable to laboratory medicine (including point-of-care testing) are particularly encouraged, even if preliminary or small scale.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


