仿生水凝胶平台显示从视网膜色素上皮到光感受器的主动力转导。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
在眼睛中,视网膜色素上皮(RPE)维持视网膜光感受器的功能和福利,并与光感受器外段(POSs)形成紧密的互锁结构。RPE与视网膜的相互作用很难在体外重现,这限制了RPE对视网膜维持功能的研究。为了克服这一挑战,我们使用涂有Matrigel的软聚丙烯酰胺水凝胶构建了一个视网膜模拟结构。将该结构引入RPE细胞的顶侧,体外模拟RPE-视网膜界面。结果,RPE细胞在培养过程中附着在水凝胶上,从而可以通过流变学和牵引力显微镜进一步研究RPE水凝胶之间的细胞粘附和力传导。这些方法被应用于视网膜和RPE之间的一个关键的相互作用过程:吞噬老化的POSs尖端,使其更新。在吞噬过程中,RPE细胞对POS颗粒施加了相当大的牵引力。力的产生是肌动蛋白依赖的,通过破坏RPE的肌动蛋白细胞骨架,力显著降低。这些结果为RPE与神经视网膜之间的多种相互作用机制提供了新的层次,为进一步研究RPE与视网膜的相互作用铺平了道路。意义说明:在眼睛中,感光神经视网膜与底层上皮组织(视网膜色素上皮,RPE)相互作用并共同起作用。这种物理相互作用的损害会导致视网膜变性和失明。然而,目前我们缺少RPE和视网膜的细胞培养模型系统,在那里这种相互作用可以被操纵和研究。为了解决这个问题,我们开发了一种以RPE细胞培养的水凝胶为基础的视网膜模拟结构。该平台能够研究界面,特别是RPE和视网膜感光细胞之间的基本更新过程。我们首次提供了直接证据,证明这一过程涉及RPE和视网膜之间的显著力传递。这些发现揭示了以前未被认识的神经细胞和上皮细胞之间的机械生物学相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
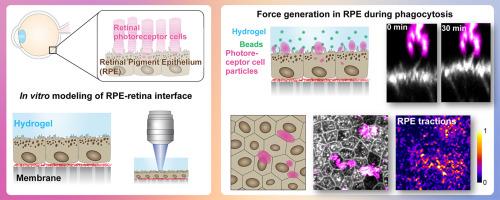
Biomimetic hydrogel platform reveals active force transduction from retinal pigment epithelium to photoreceptors
In the eye, the retinal pigment epithelium (RPE) maintains the functionality and welfare of retinal photoreceptors and forms a tight, interlocked structure with photoreceptor outer segments (POSs). The RPE-retina interaction is difficult to recapitulate in vitro, limiting the studies addressing the retinal maintenance functions of the RPE. To overcome this challenge, we constructed a retina-mimicking structure using a soft polyacrylamide hydrogel coated with Matrigel. This structure was introduced to RPE cells’ apical side to model the RPE-retina interface in vitro. As a result, RPE cells attached to the hydrogels during culture, enabling further studies of cell adhesion and force transduction between the RPE-hydrogel with rheology and traction force microscopy. These methods were applied to a critical interactive process between the retina and the RPE: phagocytosis of the aged tips of POSs enabling their renewal. During phagocytosis, RPE cells imposed considerable traction forces to the POS particles. The force generation was actin-dependent, and the forces were significantly reduced by the disruption of RPE’s actin cytoskeleton. These results add another layer to the diverse interaction mechanisms between the RPE and the neural retina and pave the way for further studies of the RPE-retina interplay.
Statement of significance
In the eye, light sensing neural retina interacts and functions jointly with the underlying epithelial tissue (retinal pigment epithelium, RPE). Impairments in this physical interaction can cause retinal degeneration and blindness. However, currently we are missing cell culture model systems of the RPE and the retina, where this interaction could be manipulated and studied. To address this, we developed a hydrogel-based retina-mimicking structure cultured with RPE cells. This platform enabled studies of the interface, particularly the essential renewal process between the RPE and retinal photoreceptor cells. For the first time, we provided direct evidence that this process involves significant force transmission between the RPE and the retina. These findings uncover a previously unrecognized mechanobiological interaction between neural and epithelial cells in the eye.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


