针对性地发现绿枝中倍半萜吲哚类生物碱。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在过去的几十年里,由于其治疗价值和丰富的异喹啉生物碱,无性系植物一直受到天然产物界的特别关注。利用我们实验室收集的这些化合物的历史数据,我们建立了一个包含322种异喹啉类和其他番槐科植物代谢物的MS/MS数据库,并命名为IQAMDB。本报告描述了从绿叶栎树(英国)茎皮中提取的已知生物碱。& Diels) Verdc。利用iqamdb通知的基于特征的分子网络,通过计算机注释和分类加权进一步完善。该策略注释了30多种化合物,并简化了三种倍半萜吲哚生物碱(SIA)的分离(1-3)。通过核磁共振、TDDFT-ECD或x射线衍射的组合进行结构解析和绝对构型鉴定,确定这些化合物为greenwaylactam D(1)和greenwaylactam E(2),它们是先前报道的greenwaylactam a的Witkop Winterfeldt氧化非对映异构体,以及polyveodrine(3),揭示了SIA的前所未有的相对构型。研究了分离得到的化合物对金黄色葡萄球菌、铜绿假单胞菌、白色念珠菌、海洋分枝杆菌的抗菌和抗真菌活性以及对寨卡病毒的抗病毒活性。聚维黄碱对海洋分枝杆菌表现出中等的抗细菌活性,而绿维内酰胺D(1)在非细胞毒性浓度下对寨卡病毒表现出中等的抗病毒活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
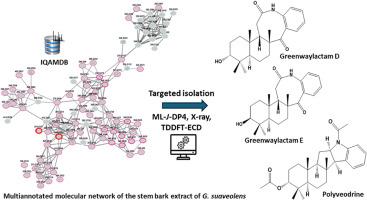
Targeted discovery of sesquiterpene indole alkaloids from Greenwayodendron suaveolens
Throughout the past decades, annonaceous plants have been of particular interest to the natural product community because of their therapeutic value and their richness in isoquinoline alkaloids. Taking advantage from our laboratory historical collection of these compounds, a MS/MS database of 322 isoquinolines and other metabolites from Annonaceae was implemented and named IQAMDB. The present report describes the dereplication of known alkaloids from stem barks of Greenwayodendron suaveolens (Engl. & Diels) Verdc. leveraging IQAMDB-informed feature-based molecular networking further refined by in silico annotation and taxonomic weighting. This strategy annotated over 30 compounds and streamlined the isolation of three sesquiterpene indole alkaloids (SIA) (1–3). Structure elucidation and absolute configuration assignment by a combination of NMR, TDDFT-ECD or X-ray diffraction determined these compounds to be greenwaylactam D (1) and greenwaylactam E (2), previously undescribed Witkop-Winterfeldt oxidized diastereoisomers of the previously reported greenwaylactam A, and polyveodrine (3), that discloses an unprecedented relative configuration for a SIA. The isolated compounds were evaluated for their antibacterial and antifungal activities against Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Mycobacterium marinum as well as their antiviral activity against Zika virus. Polyveodrine exhibited moderate antimycobacterial activity against M. marinum, whereas greenwaylactam D (1) demonstrated moderate antiviral activity against Zika virus under non-cytotoxic concentrations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


