DHYZ调节海马胆碱能通路乙酰化改善大鼠缺血性脑卒中后认知缺陷。
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
缺血性脑卒中是一种严重的脑血管疾病,常伴有退行性感觉运动障碍和持续性认知障碍,严重影响患者的生活质量。DHYZ是一种传统的中草药配方,在恢复脑缺血区域的神经功能方面显示出显著的疗效,但其改善脑卒中后认知障碍的潜力尚不充分。本研究采用大脑中动脉闭塞/再灌注(MCAO/R)模型模拟人类缺血性脑卒中的病理过程。我们通过行为学评估(Morris水迷宫)、组织病理学评估、分子分析(定量PCR、免疫荧光、Western blotting)和乙酰胆碱(ACh)含量检测相结合的方法,系统探讨DHYZ对脑卒中后认知功能障碍的治疗作用及其机制。实验数据显示,DHYZ治疗显著减少MCAO/R大鼠脑梗死体积,促进神经功能恢复,增强空间学习/记忆能力。DHYZ增强MCAO/R大鼠海马胆碱能回路的激活,通过增加CREB磷酸化,促进CREB结合蛋白(CBP)表达上调,进一步提高海马乙酰胆碱转移酶(ChAT)和乙酰胆碱酯酶(AChE)基因启动子乙酰化水平。这种级联增强了海马通路中胆碱乙酰转移酶(ChAT)和乙酰胆碱酯酶(AChE)基因启动子的乙酰化。ChAT和AChE对ACh的影响相互平衡,促进认知功能。我们的研究结果阐明了涉及胆碱能信号通路调节的新机制,并为开发针对缺血性卒中相关认知功能障碍的有效干预措施提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
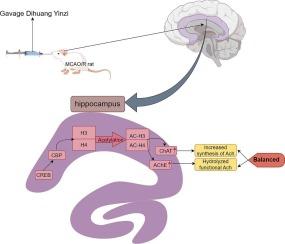
DHYZ modulates hippocampal cholinergic pathway acetylation to ameliorate cognitive deficits post-ischemic stroke in rats
Ischemic stroke is a serious cerebrovascular disease that is often accompanied by debilitating sensorimotor deficits and persistent cognitive deficits, which seriously affect patients’ quality of life. DHYZ, a traditional Chinese herbal formula, has shown significant efficacy in restoring neurological function in ischemic regions of the brain, but its potential for improving poststroke cognitive impairment remains underdeveloped. In this study, the middle cerebral artery occlusion/reperfusion (MCAO/R) model was used to reproduce the pathological process of ischemic stroke in humans. We systematically investigated the therapeutic effect of DHYZ on poststroke cognitive dysfunction and the underlying mechanisms through a combination of behavioral assessment (Morris water maze), histopathological evaluation, molecular analysis (quantitative PCR, immunofluorescence, and Western blotting), and acetylcholine (ACh) content detection. The experimental data revealed that DHYZ treatment significantly reduced the volume of cerebral infarction, improved neurological recovery, and enhanced spatial learning/memory ability in MCAO/R rats. DHYZ enhanced the activation of hippocampal cholinergic circuits in MCAO/R rats, promoted the upregulation of CREB-binding protein (CBP) expression by increasing CREB phosphorylation and further increased the acetylation levels of the promoters of the acetylcholine transferase (ChAT) and acetylcholinesterase (AChE) genes in the hippocampus. This cascade enhances acetylation of the choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) gene promoters in the hippocampal pathway. The effects of ChAT and AChE on ACh balance each other and promote cognitive function. Our findings elucidate a novel mechanism involving the regulation of cholinergic signaling pathways and provide new insights for the development of effective interventions targeting ischemic stroke-related cognitive dysfunction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


