水热原位合成Mg/Al-LDHs对混合重金属的可持续修复
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究探讨了水热法和原位法合成镁铝/层状双氢氧化物(Mg/Al-LDHs)同时去除水溶液中Cu(II)、Pb(II)和Cr(VI)的比较效果。采用SEM、XRD、FTIR、BET表面积分析、XPS和zeta电位等手段对吸附前后的LDHs进行了表征。结果表明,水热合成的Mg/Al-LDHs具有增强的结构有序性和优异的结晶度,这可以从尖锐而强烈的XRD峰和增大的BET比表面积中得到证明,并且具有更高的吸附能力,对Cu(II)的吸附量为240 Mg/ g,对Pb(II)的吸附量为512 Mg/ g,对Cr(VI)的吸附量为484 Mg/ g,在60 min内达到平衡。吸附动力学遵循准二阶模型,Langmuir等温线最能描述平衡数据,表明其为单层化学吸附过程。相比之下,原位合成方法在操作简单和试剂效率方面具有优势,在碱性条件下可以达到100%的Pb(II)和80%的Cu(II)和Cr(VI)的去除率。机理分析表明,吸附过程包括表面络合、离子交换和表面沉淀。此外,Cu(II)的吸附仅受pH变化的轻微影响,在较高的pH水平下观察到容量略有增加。随着pH的升高,Pb(II)的吸附量明显增加,而Cr(VI)的吸附量则相反,随着pH的升高,其吸附量减少。这些结果表明,优化LDH矿物结构可以通过提高稳定性和表面反应性来增强重金属的吸收,从而支持开发高性能、可重复使用的吸附剂,用于可持续的废水修复。本文章由计算机程序翻译,如有差异,请以英文原文为准。
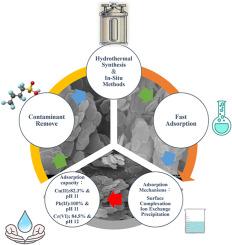
Sustainable remediation of mixed heavy metals using hydrothermal and in-situ synthesis of Mg/Al-LDHs
This study explores the comparative efficacy of magnesium-aluminum/layered double hydroxides (Mg/Al-LDHs) synthesized via hydrothermal and in situ methods for the simultaneous removal of Cu(II), Pb(II), and Cr(VI) from aqueous solutions. The LDHs before and after adsorption were thoroughly characterized using SEM, XRD, FTIR, BET surface area analysis, XPS, and zeta potential measurements. The results demonstrated that hydrothermally synthesized Mg/Al-LDHs exhibited enhanced structural ordering and superior crystallinity, as evidenced by sharp and intense XRD peaks and increased BET surface area, and higher adsorption capacities, 240 mg/g for Cu(II), 512 mg/g for Pb(II), and 484 mg/g for Cr(VI), achieving equilibrium within 60 min. Adsorption kinetics followed a pseudo-second-order model, and equilibrium data were best described by the Langmuir isotherm, indicating a monolayer chemisorption process. In contrast, the in situ synthesis method proved advantageous in operational simplicity and reagent efficiency, achieving 100 % Pb(II) and >80 % Cu(II) and Cr(VI) removal under alkaline conditions. Mechanistic analysis suggested that adsorption involved surface complexation, ion exchange, and surface precipitation. Furthermore, the adsorption of Cu(II) was only marginally affected by changes in pH, with a slight increase in capacity observed at higher pH levels. In contrast, Pb(II) showed a pronounced rise in adsorption with increasing pH, while Cr(VI) displayed the opposite behavior, with its adsorption decreasing as pH increased. These results demonstrate that optimizing LDH mineral structure enhances heavy metal uptake through improved stability and surface reactivity, supporting the development of high-performance, reusable adsorbents for sustainable wastewater remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


