苯甲醛和2-氨基苯甲酸在对甲苯磺酸催化下直接合成2-(苄基氨基)苯甲酸
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
羰基化合物的直接还原胺化反应是合成胺类衍生物的一种很有前途的方法。通常,化学计量还原剂会产生大量的废物,其中一些是有害的,难以处理。因此,利用对环境污染低、有害副产物最少的还原剂合成胺是非常理想的。本研究发现,在对甲苯磺酸(TsOH)的催化下,取代苯甲醛和取代2-氨基苯甲酸可以在一锅法中直接转化为取代2-(苄基氨基)苯甲酸,唯一的副产物是取代苯甲酸。研究了反应机理,结果表明:在TsOH催化下,苯甲醛还原希夫碱的碳氮双键,生成2-(苄基氨基)苯甲酸。这种现象以前没有观察到。该方法的优点是反应条件温和,不需要金属催化,也不需要额外的还原剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
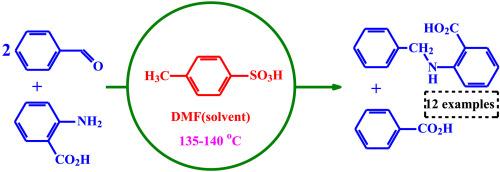
Direct synthesis of 2-(benzylamino)benzoic acids by using benzaldehydes and 2-aminobenzoic acids with catalysis of p-toluenesulfonic acid
Direct reductive amination of carbonyl compounds is a promising method for the synthesis of amine derivatives. Usually, stoichiometric reducing agents generate significant waste products, some of which are hazardous and difficult to handle. Therefore, it is highly desirable to synthesize amines using reducing agents that cause low environmental pollution and minimize hazardous by-products. In this work, It was discovered that substituted benzaldehydes and substituted 2-aminobenzoic acids can be directly converted into substituted 2-(benzylamino)benzoic acids using p-toluenesulfonic acid (TsOH) as a catalyst in a one-pot method, where the only by-product is substituted benzoic acid. The reaction mechanism was investigated, and the results showed that the Schiff base's carbon-nitrogen double bond is reduced by benzaldehyde under TsOH catalysis, resulting in 2-(benzylamino)benzoic acid. This phenomenon was not observed previously. The advantage of this method lies in its mild reaction conditions, as it requires neither metal catalysis nor additional reducing agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


