基于甘油的软囊系统用于鼻至脑输送131i -褪黑素:合成、表征、生物分布和药代动力学研究
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-03
DOI:10.1016/j.jddst.2025.107482
引用次数: 0
摘要
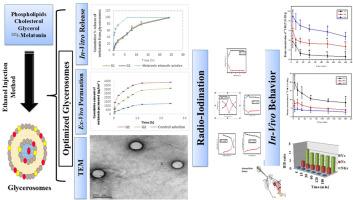
最近,甘油已经显示出一些药物的优点,特别是在鼻内脑输送。在目前的手稿中,先进的甘油为基础的软囊系统(甘油小体,Gs)包含褪黑激素(MLT),松果体激素,在这方面制定和表征。对制备的囊泡进行了粒径(PS)、ζ电位(ZP)、包封率(EE%)和储存稳定性的测试。并对所选制剂进行药物释放模式、表面形貌、黏度、pH值测定及通过羊鼻黏膜的通透性测定。采用放射性碘化mlt (131I-MLT)进行生物分布和药代动力学研究,以评估静脉注射溶液(Vs)、鼻内溶液(Ns)和鼻内给予甘油小体(ng)的体内行为。结果表明,负载mlt的囊泡的PS值为202 ~ 538 nm,负ZP值为- 13.10 ~ - 19.26 mV, EE%值为84.21 ~ 96.53%,胶体性能变化不大,具有良好的稳定性。此外,优化后的配方呈球形,24 h内的药物释放时间达到≈100%,并且相对于MLT乙醇溶液的渗透性提高了约4倍。体内实验结果显示,与131I-MLT相比,NGs在前5min的脑血比(B/B)分别是Vs和Ns的11.0和2.8倍。因此,结果证明了使用Gs作为鼻内脑内MLT传递的安全可靠平台的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Glycerol-based soft vesicular systems for nose to brain delivery of 131I-melatonin: Synthesis, characterization, biodistribution and Pharmacokinetic investigations
Recently, glycerol has shown several pharmaceutical merits, particularly in intranasal brain delivery. In the present manuscript, advanced glycerol-based soft vesicular systems (glycerosomes, Gs) encompassing melatonin (MLT), a pineal gland hormone, were formulated and characterized in this regard. The prepared vesicles were tested for their particle size (PS), zeta potential (ZP), encapsulation efficiency (EE%) as well as storage stability. Besides, the drug release pattern, surface morphology, viscosity and pH measurements, and permeability through sheep nasal mucosa were conducted on the selected preparations. Bio-distribution along with pharmacokinetic investigations were conducted employing the radioiodinated-MLT (131I-MLT) to assess the in-vivo behavior of intravenous solutions (Vs), intranasal solutions (Ns), and intranasal given glycerosomes (NGs). Results revealed that MLT-loaded vesicles showed PS ranging from 202 to 538 nm, negative ZP values varying from −13.10 to −19.26 mV, superior EE% values possessing a range of 84.21–96.53 % and good stability properties manifested by insignificant change in their colloidal properties. In addition, the optimized formulation(s) displayed spherical shape, prolonged drug release reaching ≈100 % throughout 24 h and augmented permeation of approximately 4-fold higher relative to MLT ethanolic solution. The findings of the in-vivo study showed that during the first 5 min, brain/blood ratio (B/B) of NGs was 11.0 and 2.8 times greater compared to Vs and Ns of 131I-MLT, respectively. Accordingly, the results demonstrated the possibility of employing Gs as a secure and reliable platform for MLT intranasal brain delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


