左西替利嗪经经肾上腺体热敏原位凝胶鼻内递送:变应性鼻炎中增强鼻腔吸收和抗组胺作用的有希望的策略
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-04
DOI:10.1016/j.jddst.2025.107452
引用次数: 0
摘要
近年来,变应性鼻炎的发病率不断上升,需要实施更有效和安全的治疗策略。左西替利嗪(LVC)是第二代抗组胺药,用于变应性鼻炎、过敏性结膜炎、特应性和接触性皮炎、湿疹和荨麻疹。本研究首次在动物模型实验中提出经酶体热敏原位凝胶作为鼻内给药系统治疗AR。采用薄膜水合法制备了负载lvc的转酶体(LVC-TEs)。然后评估zeta电位、粒径、多分散性指数和捕获效率(EE %)。此外,对优化后的LVC-TE配方(TE1)进行了TEM和FTIR研究。优化后的配方集成到基于poloxam407和188的热敏原位凝胶中,羟丙基甲基纤维素作为粘接聚合物(TE1-ISG)。TE1-ISG的评估包括评估各种参数和体外渗透。TE1纳米颗粒呈光滑球形,EE%最高(92.58±1.21),粒径最高(130.64±2.10 nm),多分散性指数最高(0.46±0.04),zeta电位最高(19.52±0.52 mV)。TE1的释放谱表现为初始爆发,然后在24 h内持续释放。通过卵清蛋白致敏后鼻腔刺激,在24只大鼠实验中诱导AR。ELISA法检测各组大鼠血清IgE、组胺、PAF、IL-1β、IL-5水平及鼻黏膜PAF、LTB4水平。采用qRT-PCR分析PGD2和CCL24 mRNA的表达。通过组织病理学和免疫组化评价TGF-β和FOXO1的表达。本研究表明,开发的TE1-ISG鼻内给药提供了一个有效的传递平台,在治疗ova诱导的大鼠鼻炎中显示出积极的药效学效应,代表了一种新的、有前景的AR治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
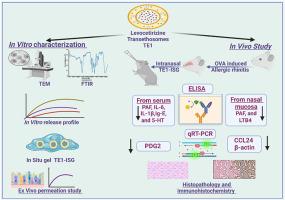
Intranasal delivery of levocetirizine via transethosomal thermosensitive in situ gel: A promising strategy for enhanced nasal absorption and antihistamine action in allergic rhinitis
Allergic rhinitis has shown a rising prevalence throughout the years, necessitating the implementation of more effective and safe treatment strategies. Levocetirizine (LVC) is an antihistamine of the second generation used for Allergic Rhinitis, Allergic Conjunctivitis, atopic and contact dermatitis, eczema, and urticaria. This study proposed a transethosomal thermosensitive in-situ gel as an intranasal delivery system experimentally for the first time to treat AR in animal models. LVC-loaded transethosomes (LVC-TEs) were prepared using the thin film hydration method. The zeta potential, particle size, polydispersity index, and entrapment efficiency (EE %) were then evaluated. Additionally, TEM and FTIR investigations were performed on the optimized LVC-TE formulation (TE1). The optimized formula integrates into a thermosensitive in-situ gel based on poloxamer 407 and 188, and hydroxypropyl methylcellulose as a mucoadhesive polymer (TE1-ISG). The evaluation of TE1-ISG involved assessing various parameters, and ex-vivo permeation. TE1 displayed smooth spherical nanoparticles with the highest EE% (92.58 ± 1.21), optimum particle size (130.64 ± 2.10 nm), polydispersity index (0.46 ± 0.04), and zeta potential (19.52 ± 0.52 mV). The release profile of TE1 displayed an initial burst followed by sustained release within 24 h. AR was experimentally induced in 24 rats through ovalbumin sensitization followed by nasal challenges. Serum levels of IgE, histamine, PAF, IL-1β, and IL-5 were measured using ELISA, along with PAF and LTB4 levels in the nasal mucosa. The expression of PGD2 and CCL24 mRNA was analyzed via qRT-PCR. Histopathological and immunohistochemical evaluations were performed to assess TGF-β and FOXO1 expression. This study demonstrates that the developed intranasal TE1-ISG provides an effective delivery platform, showing positive pharmacodynamics effects in treating OVA-induced rhinitis in rats, and represents a novel and promising strategy for AR treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


