多诺瓦利什曼原虫改变宿主鞘脂生物合成途径调控microRNA hsa-miR-15a-5p以维持其生存
IF 3.5
3区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
多诺瓦利什曼原虫是一种细胞内原生动物寄生虫,已经成功地进化到操纵宿主巨噬细胞。利什曼原虫逃避巨噬细胞功能的确切机制尚不完全清楚。最近,一些研究表明,病原体以宿主microrna为靶点,改变细胞通路以维持其生存。本研究探讨了多诺瓦利什曼原虫感染过程中宿主鞘脂生物合成途径调控microrna的变化。鞘脂生物合成途径基因包括丝氨酸棕榈酰转移酶长链碱基1 (SPTLC1)、3-酮二氢鞘脂还原酶(KDSR)、神经酰胺合成酶1(CERS1)和二氢神经酰胺去饱和酶1(DEGS1)上调,而n -酰基鞘氨酸氨基水解酶1(ASAH1)下调,但在利什曼原虫感染THP-1衍生的巨噬细胞(TDM)中,24小时鞘磷脂合成酶1(SGMS1)和鞘氨酸激酶1(SPHK1)未观察到显著变化。使用miRWalk 2.0的生物信息学分析预测SPTLC1是hsa-miR-15a-5p和hsa-miR-330-5p的靶标。CERS1被hsa-miR-10396a-3p靶向,ASAH1被hsa-miR-513a-5p靶向;所有这些mirna在感染期间都被报道失调。由于在Targetscan、miRDB和miRTarBase三个数据库中发现hsa-miR-15a-5p共同靶向SPTLC1,因此选择hsa-miR-15a-5p的表达进行进一步研究。我们发现hsa-miR-15a-5p在多诺瓦利什曼原虫感染期间表达下调。hsa-miR-15a-5p的计算机靶标预测和体外靶标验证表明SPTLC1是靶标之一。此外,hsa-miR-15a-5p的模拟物降低了SPTLC1的表达,主要上调了促炎细胞因子,并减少了TDM和外周血单核细胞(PBMC)来源的人巨噬细胞中的寄生虫。本文章由计算机程序翻译,如有差异,请以英文原文为准。
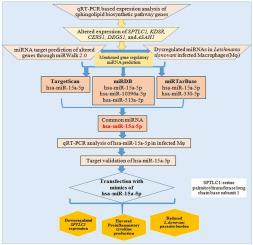
Leishmania donovani alters the host sphingolipid biosynthetic pathway regulatory microRNA hsa-miR-15a-5p for its survival
Leishmania donovani is an intracellular protozoan parasite that has successfully evolved to manipulate host macrophages. The exact mechanism by which Leishmania spp evades macrophage function is not fully understood. Recently, several studies have shown that pathogens target host-microRNA to alter cellular pathways for their persistence. Here, we explored the alterations in host sphingolipid biosynthetic pathway regulatory microRNAs during Leishmania donovani infection. Here, the sphingolipid biosynthetic pathway genes serine palmitoyltransferase long chain base subunit 1 (SPTLC1), 3-ketodihydrosphingosine reductase (KDSR), ceramide synthase 1(CERS1) and dihydroceramide desaturase 1 (DEGS1) were found to be upregulated while N-Acylsphingosine Amidohydrolase 1 (ASAH1) was downregulated but no significant changes were observed in sphingomyelin synthase 1 (SGMS1) and sphingosine kinase 1 (SPHK1) in Leishmania donovani infected THP-1 derived macrophages (TDM) at 24 h. Bioinformatic analysis using miRWalk 2.0 predicted SPTLC1 to be a target of hsa-miR-15a-5p and hsa-miR-330-5p, CERS1 to be targeted by hsa-miR-10396a-3p, and ASAH1 by hsa-miR-513a-5p; all of these miRNAs have been previously reported to be dysregulated during infection. Since hsa-miR-15a-5p was found common to target SPTLC1 in all three databases, namely Targetscan, miRDB, and miRTarBase therefore the expression of hsa-miR-15a-5p was selected for further studies. We found a downregulated expression of hsa-miR-15a-5p during Leishmania donovani infection. In silico target prediction followed by in vitro target validation of hsa-miR-15a-5p showed SPTLC1 as one of the targets. Additionally, mimics of hsa-miR-15a-5p reduced the expression of SPTLC1, upregulated mainly the proinflammatory cytokines, and reduced the parasites in TDM as well as Peripheral Blood Mononuclear Cell (PBMC) derived human macrophages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microbial pathogenesis
医学-免疫学
CiteScore
7.40
自引率
2.60%
发文量
472
审稿时长
56 days
期刊介绍:
Microbial Pathogenesis publishes original contributions and reviews about the molecular and cellular mechanisms of infectious diseases. It covers microbiology, host-pathogen interaction and immunology related to infectious agents, including bacteria, fungi, viruses and protozoa. It also accepts papers in the field of clinical microbiology, with the exception of case reports.
Research Areas Include:
-Pathogenesis
-Virulence factors
-Host susceptibility or resistance
-Immune mechanisms
-Identification, cloning and sequencing of relevant genes
-Genetic studies
-Viruses, prokaryotic organisms and protozoa
-Microbiota
-Systems biology related to infectious diseases
-Targets for vaccine design (pre-clinical studies)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


