发现新型茚唑衍生物Ⅰ型PRMTs抑制剂治疗三阴性乳腺癌
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
I型蛋白精氨酸甲基转移酶(PRMTs)在包括癌症在内的多种疾病中发挥重要作用。I型PRMTs的抑制显著抑制乳腺癌的生长,特别是三阴性乳腺癌(TNBC)。开发高效、选择性的I型PRMTs抑制剂已成为近年来的研究热点。在本研究中,通过支架跳跃策略设计了一系列六元融合五元杂环衍生物。通过结构优化,得到吡唑衍生物B9,即SKLB06329。该化合物对I型PRMT5/7及多种赖氨酸甲基转移酶均表现出纳摩尔至低纳摩尔水平的抑制活性。SKLB06329能显著抑制TNBC细胞增殖,诱导细胞凋亡,抑制细胞内不对称二甲基精氨酸(ADMA)的表达。当静脉注射时,它表现出良好的药代动力学特性。SKLB06329可以作为有效的先导化合物进一步研究,为TNBC的治疗提供新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

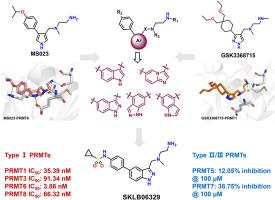
Discovery of novel indazole derivatives as type Ⅰ PRMTs inhibitors for the treatment of triple-negative breast cancer
Type I protein arginine methyltransferases (PRMTs) play significant roles in various diseases, including cancer. The inhibition of type I PRMTs significantly suppresses the growth of breast cancer, particularly triple-negative breast cancer (TNBC). The development of potent and selective type I PRMTs inhibitors has become a research hotspot in recent years. In this study, a series of six-membered fused five-membered heterocyclic derivatives were designed via a scaffold hopping strategy. Through structural optimization, the pyrazole derivative B9, namely SKLB06329, was obtained. This compound exhibited inhibitory activity against Type I PRMTs at the nanomolar to low nanomolar level and showed good selectivity for PRMT5/7 and various lysine methyltransferases. SKLB06329 could significantly inhibit the proliferation of TNBC cells, induce apoptosis, and suppress the expression of asymmetric dimethylarginine (ADMA) within cells. When administered intravenously, it demonstrated favorable pharmacokinetic properties. SKLB06329 can serve as an effective lead compound for further research, providing a new strategy for the treatment of TNBC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


