2,6 -二取代吲哚衍生物作为METTL3抑制剂的设计、合成和生物学评价
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
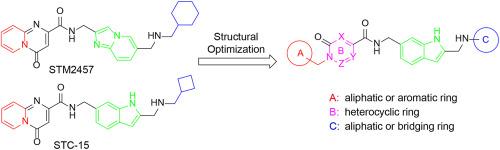
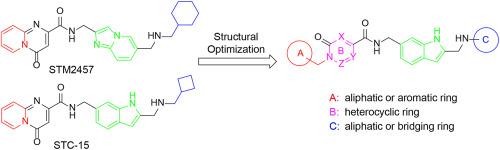
METTL3 (Methyltransferase Like 3)由于其在RNA甲基化修饰中的关键作用,近年来引起了人们的广泛关注,成为治疗癌症的一种新的、极具前景的治疗靶点。在这里,我们报道了2,6 -二取代吲哚衍生物作为METTL3抑制剂的优化和评价。代表性化合物16e对METTL3的IC50值为0.49±0.30 nM。分子动力学模拟证实了16e与METTL3的结合。在体外实验中,16e显著降低急性髓性白血病MOLM-13细胞和卵巢癌SKOV3细胞中的m6A水平,显著抑制细胞增殖,诱导细胞凋亡,抑制细胞迁移,下调m6A下游靶基因c-MYC和BCL2的表达。此外,化合物16e在SKOV3异种移植瘤模型中表现出良好的药动学特征和抗肿瘤效果。总的来说,这项研究为开发新的癌症治疗方法提供了一个有希望的候选化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis, and biological evaluation of 2, 6-di-substituted indole derivatives as METTL3 inhibitors
METTL3 (Methyltransferase Like 3) has garnered significant attention in recent years due to its pivotal role in RNA methylation modification, emerging as a novel and highly promising therapeutic target for the treatment of cancer. Here, we report the optimization and evaluation of 2, 6-di-substituted indole derivatives as METTL3 inhibitors. The representative compound 16e showed an IC50 value of 0.49 ± 0.30 nM against METTL3. Molecular dynamics simulation confirmed that the binding of 16e to METTL3. In vitro assays, 16e significantly reduced m6A levels in acute myeloid leukemia MOLM-13 cells and ovarian cancer SKOV3 cells, markedly inhibited cell proliferation, induced cells apoptosis, suppressed cells migration, and downregulated the expression of m6A downstream target genes c-MYC and BCL2. Additionally, compound 16e showed favorable pharmacokinetic characteristics and good antitumor efficacy in a SKOV3 xenograft model. Collectively, this study provides a promising candidate compound for the development of new therapeutics for cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


