R-P相高熵钙钛矿la0.5 sr1.5 mn0.2 fe0.2 ni0.2 cu0.2 co0.2 2o4通过多途径活化实现PMS的完全利用,实现高效水净化
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
传统钙钛矿和Ruddlesden-Popper (R-P)相钙钛矿已经在基于过氧单硫酸盐(PMS)的高级氧化工艺(AOPS)中进行了广泛的研究,但它们的实际应用仍然受到长期稳定性、多途径活化能力和过量金属浸出的限制。高熵钙钛矿(HEPs)具有熵稳定的结构完整性和多金属活性位点,为解决这些挑战提供了一个有希望的解决方案。本研究采用R-P相高熵钙钛矿(La0.5Sr1.5Mn0.2Fe0.2Ni0.2Cu0.2Co0.2O4, LSMFNCC)研究了PMS反应的多途径激活机制。在LSMFNCC/PMS系统中,自由基途径(OH, SO4−和O2−)和非自由基途径(1O2, Fe(IV) = O和电子转移途径(ETP))共同促进左氧氟沙星(LVFX)的去除。这种协同多途径激活以最小的催化剂用量(0.05 g/L)实现了卓越的性能,在30 min内实现了接近定量的PMS利用率(≈100 %)和大约95 %的LVFX去除率。效率的提高源于多金属活性位点的个体和协同效应,加上由熵驱动的晶格畸变产生的富氧空位(OVs)缺陷结构,它们共同提高了PMS活化和污染物降解过程中的电子转移效率。值得注意的是,LSMFNCC在各种条件下都表现出了出色的稳定性,包括不同的阴离子、阳离子、pH值和真实的水基质,这一点已经通过连续五次的回收测试得到了证实。利用T.E.S.T软件进行最小金属浸出和毒性评价,进一步验证了其环境相容性。这些发现确立了LSMFNCC作为高性能pms活化催化剂的地位,同时为水处理应用中hep的设计提供了基础见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
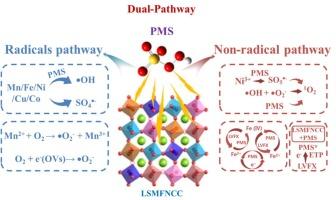
R-P phase high-entropy perovskite La0.5Sr1.5Mn0.2Fe0.2Ni0.2Cu0.2Co0.2O4 achieves complete PMS utilization via multi-pathway activation for efficient water purification
Both conventional and Ruddlesden-Popper (R-P) phase perovskites have been extensively investigated in peroxymonosulfate (PMS)-based advanced oxidation processes (AOPS), yet their practical application remains constrained by limitations in long-term stability, multi-pathway activation capability, and excessive metal leaching. High-entropy perovskites (HEPs), with their entropy-stabilized structural integrity and multi-metallic active sites, offer a promising solution to these challenges. In this study, an R-P phase high-entropy perovskite (La0.5Sr1.5Mn0.2Fe0.2Ni0.2Cu0.2Co0.2O4, LSMFNCC) was employed to investigate the multi-pathway activation mechanisms in PMS reactions. Within the LSMFNCC/PMS system, both radical pathways ( OH, SO4
OH, SO4 −, and
−, and  O2−) and non-radical pathways (1O2, Fe(IV) = O, and electron transfer pathway (ETP)) collectively contributed to Levofloxacin (LVFX) removal. This synergistic multi-pathway activation enabled exceptional performance with minimal catalyst dosage (0.05 g/L), achieving near-quantitative PMS utilization (≈100 %) and approximately 95 % LVFX removal within 30 min. The enhanced efficiency stems from the individual and cooperative effects of multi-metallic active sites, coupled with oxygen vacancies (OVs)-rich defect structures generated through entropy-driven lattice distortion, which collectively improve electron transfer efficiency during both PMS activation and pollutant degradation. Notably, LSMFNCC demonstrated remarkable stability across diverse conditions, including varying anions, cations, pH levels, and real water matrices, as confirmed through five consecutive recycling tests. Minimal metal leaching and toxicity evaluation using T.E.S.T software further verified its environmental compatibility. These findings establish LSMFNCC as a high-performance PMS-activating catalyst while providing fundamental insights for designing HEPs in water treatment applications.
O2−) and non-radical pathways (1O2, Fe(IV) = O, and electron transfer pathway (ETP)) collectively contributed to Levofloxacin (LVFX) removal. This synergistic multi-pathway activation enabled exceptional performance with minimal catalyst dosage (0.05 g/L), achieving near-quantitative PMS utilization (≈100 %) and approximately 95 % LVFX removal within 30 min. The enhanced efficiency stems from the individual and cooperative effects of multi-metallic active sites, coupled with oxygen vacancies (OVs)-rich defect structures generated through entropy-driven lattice distortion, which collectively improve electron transfer efficiency during both PMS activation and pollutant degradation. Notably, LSMFNCC demonstrated remarkable stability across diverse conditions, including varying anions, cations, pH levels, and real water matrices, as confirmed through five consecutive recycling tests. Minimal metal leaching and toxicity evaluation using T.E.S.T software further verified its environmental compatibility. These findings establish LSMFNCC as a high-performance PMS-activating catalyst while providing fundamental insights for designing HEPs in water treatment applications.
 OH, SO4
OH, SO4 −, and
−, and  O2−) and non-radical pathways (1O2, Fe(IV) = O, and electron transfer pathway (ETP)) collectively contributed to Levofloxacin (LVFX) removal. This synergistic multi-pathway activation enabled exceptional performance with minimal catalyst dosage (0.05 g/L), achieving near-quantitative PMS utilization (≈100 %) and approximately 95 % LVFX removal within 30 min. The enhanced efficiency stems from the individual and cooperative effects of multi-metallic active sites, coupled with oxygen vacancies (OVs)-rich defect structures generated through entropy-driven lattice distortion, which collectively improve electron transfer efficiency during both PMS activation and pollutant degradation. Notably, LSMFNCC demonstrated remarkable stability across diverse conditions, including varying anions, cations, pH levels, and real water matrices, as confirmed through five consecutive recycling tests. Minimal metal leaching and toxicity evaluation using T.E.S.T software further verified its environmental compatibility. These findings establish LSMFNCC as a high-performance PMS-activating catalyst while providing fundamental insights for designing HEPs in water treatment applications.
O2−) and non-radical pathways (1O2, Fe(IV) = O, and electron transfer pathway (ETP)) collectively contributed to Levofloxacin (LVFX) removal. This synergistic multi-pathway activation enabled exceptional performance with minimal catalyst dosage (0.05 g/L), achieving near-quantitative PMS utilization (≈100 %) and approximately 95 % LVFX removal within 30 min. The enhanced efficiency stems from the individual and cooperative effects of multi-metallic active sites, coupled with oxygen vacancies (OVs)-rich defect structures generated through entropy-driven lattice distortion, which collectively improve electron transfer efficiency during both PMS activation and pollutant degradation. Notably, LSMFNCC demonstrated remarkable stability across diverse conditions, including varying anions, cations, pH levels, and real water matrices, as confirmed through five consecutive recycling tests. Minimal metal leaching and toxicity evaluation using T.E.S.T software further verified its environmental compatibility. These findings establish LSMFNCC as a high-performance PMS-activating catalyst while providing fundamental insights for designing HEPs in water treatment applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


