配体诱导的锆基吸附剂的电子和几何调制对氟和亚砷酸盐的有效去污
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
锆基材料对F−和As(III)具有较强的亲和力,但其水解敏感性和对As(III)的有限吸附能力阻碍了其实际应用。本研究采用配体修饰策略,将五种有机配体(乙酰丙酮、丙二酸、乙酸、柠檬酸和酒石酸)通过溶胶-凝胶法合成结构稳定的锆基吸附剂(PZA)。系统地研究了F−和As(III)的吸附性能及其去除机理。我们的研究结果表明,配体修饰显著优化了材料的物理化学性质,提高了吸附性能。柠檬酸改性的吸附剂对F−的吸附量为79 mg/g,比未改性的材料增加了1.4倍,而乙酰丙酮改性的吸附剂对As(III)的吸附量为258 mg/g,超过了大多数已报道的材料。密度功能理论计算表明,配体通过“几何构型调节”和“电子效应”双重机制改善了吸附效果:(1)配体修饰改变了材料表面的静电势分布,使正电荷区和负电荷区分别扩大了29 - 42% %和20 - 26% %,从而加强了与污染物的静电相互作用;(2)前沿轨道分析表明,配体减小了HOMO-LUMO的能隙(ΔE减小了3.0 ~ 24.6 %),增强了电子转移能力;(3)结合能计算证实了吸附过程的自发性。该研究为通过精确配体工程合理设计高性能环境功能材料提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
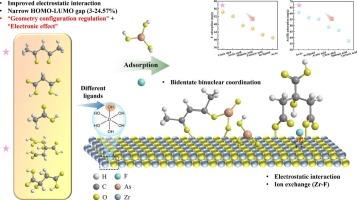
Ligand-induced electronic and geometric modulation of zirconium-based adsorbents for efficient decontamination of fluoride and arsenite
Zirconium-based materials exhibit strong affinity for F− and As(III), but their practical application is hindered by hydrolysis susceptibility and limited adsorption capacity for As(III). In this study, we employed a ligand modification strategy, incorporating five organic ligands (acetylacetone, malonic acid, acetic acid, citric acid, and tartaric acid) to synthesize structurally stabilized zirconium-based adsorbents (PZA) via the sol-gel method. The adsorption performance and underlying mechanisms for F− and As(III) removal were systematically investigated. Our results demonstrate that ligand modification significantly optimizes the physicochemical properties of the materials, enhancing adsorption performance. The citric acid-modified adsorbent achieved a F− adsorption capacity of 79 mg/g, a 1.4-fold increase over unmodified materials, whereas the acetylacetone-modified adsorbent reached an As(III) adsorption capacity of 258 mg/g, surpassing most reported materials. Density functional theory calculations reveal that ligands improve adsorption through dual mechanisms of “geometrical configuration regulation” and “electronic effect”: (1) Ligand modification alters the electrostatic potential distribution on the material surface, expanding positive and negative charge regions by 29–42 % and 20–26 %, respectively, thereby strengthening electrostatic interactions with pollutants; (2) Frontier orbital analysis indicates that ligands reduce the HOMO-LUMO energy gap (ΔE decreased by 3.0–24.6 %), enhancing electron transfer capability; (3) Binding energy calculations confirm the spontaneity of the adsorption process. This study provides a novel strategy for the rational design of high-performance environmental functional materials through precise ligand engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


