粘蛋白屏障对环肽体外膜通透性的影响。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
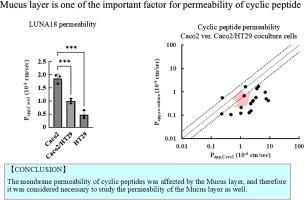
本研究利用体外实验系统研究了粘蛋白层在环肽通透性中的作用。我们利用生物类似物粘蛋白和Caco2/HT29细胞对18个环肽和参比化合物的膜通透性进行了评价。当使用生物仿制黏液蛋白时,细胞旁标记物(FD4和LY)和地高辛(一种低分子量化合物)的通透性不受黏液蛋白存在的影响。相反,环孢素A(一种市售的环肽药物)的通透性降低。在Caco2/HT29共培养细胞中,与单独培养Caco2细胞相比,大多数化合物由于间质渗透性增强而表现出渗透性增加,但环孢素A在粘蛋白存在下表现出渗透性降低。同样,在Caco2/HT29共培养和HT29细胞中,正在开发的环状肽LUNA18的通透性分别降低了54.1%和26.0%。此外,在Caco2/HT29共培养的细胞中,18个环肽中的12个表现出膜通透性降低≥2倍。这些发现表明,目前的渗透性评估方法,没有考虑粘蛋白层,可能高估了高亲脂性环肽的渗透性。粘蛋白层可能是环肽膜通透性的限速因子,强调了在环肽评估方案中包括粘蛋白层通透性的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of the mucin barrier on the in vitro membrane permeability of cyclic peptides
This study investigated the role of the mucin layer in cyclic peptide permeability using an in vitro assay system. We evaluated the membrane permeability of 18 cyclic peptides and reference compounds using biosimilar mucin and Caco-2/HT29 cells. The permeability of paracellular markers (FD4 and LY) and digoxin (a low molecular weight compound) remained unaffected by mucin presence when using biosimilar mucin. Conversely, cyclosporin A, a commercially available cyclic peptide drug, showed reduced permeability. In Caco-2/HT29 co-cultured cells compared with Caco-2 cells only, most compounds exhibited increased permeability due to enhanced interstitial permeability, except for cyclosporin A, which showed decreased permeability in the presence of mucin. Similarly, the permeability of LUNA18, a cyclic peptide under development, was reduced by 54.1 and 26.0 % in both Caco-2/HT29 co-cultured and HT29 cells. Furthermore, 12 of 18 cyclic peptides exhibited ≥ 2-fold decrease in membrane permeability in Caco-2/HT29 co-cultured cells. These findings suggest that current permeability evaluation methods, which do not account for the mucin layer, may overestimate the permeability of highly lipophilic cyclic peptides. The mucin layer may serve as a rate-limiting factor in cyclic peptide membrane permeability, highlighting the importance of including mucin layer permeability in cyclic peptide evaluation protocols.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


