富马酸二甲酯是病理性血管生成的抑制剂。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
血管内皮生长因子(VEGF)是一种促血管生成分子,在伤口愈合过程中支持血管生长,但也在致盲眼病如新生血管性年龄相关性黄斑变性(nAMD)中驱动病理性新生血管。富马酸二甲酯(DMFu)是一种fda批准的治疗多发性硬化症的药物,此前在视网膜色素上皮(一种被nAMD破坏的关键结构)中显示出有希望的抗炎特性。在这里,我们通过识别DMFu在脉络膜和视网膜内皮细胞中的抗血管生成能力来扩展DMFu的多表型治疗潜力。在小鼠脉络膜外植体发芽试验中,DMFu明显减弱了脉络膜内皮细胞的增殖。即使在VEGF存在的情况下,DMFu也会破坏人微血管视网膜内皮细胞(HRECs)的细胞迁移和管形成。大量RNA测序显示,DMFu成功改善了vegf控制基因的表达。加权基因共表达网络和基因集富集分析证实了分支血管形态发生通路的下调,但也揭示了新的转录作用机制,包括控制微管运输和atp合成耦合电子传递。测定了最大线粒体呼吸的一致下降和糖酵解和糖酵解能力的增加。western blot结果显示SDHB亚基复合物II蛋白表达降低。由于nAMD的治疗方法是有限的和侵入性的,DMFu是一个竞争者,可以迅速重新定位为口服的,对患者友好的治疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
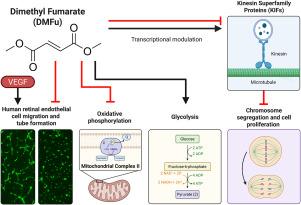
Dimethyl fumarate is an inhibitor of pathological angiogenesis
Vascular endothelial growth factor (VEGF), a pro-angiogenic molecule, supports blood vessel growth during wound healing but also drives pathological neovascularization in blinding eye diseases such as neovascular age-related macular degeneration (nAMD). Dimethyl fumarate (DMFu), an FDA-approved drug for multiple sclerosis, has shown promising anti-inflammatory properties in the retinal pigment epithelium, a crucial structure disrupted in nAMD. Here, we extend the therapeutic potential of DMFu by discerning the anti-angiogenic capabilities of DMFu in choroidal and retinal endothelial cells. Choroidal endothelial cell proliferation was significantly attenuated by DMFu in the mouse choroidal sprouting assay. Even in the presence of VEGF, DMFu disrupted cell migration and tube formation in human microvascular retinal endothelial cells (HRECs). Bulk RNA sequencing highlighted that DMFu successfully ameliorated the expression of VEGF-controlled gene expression. Weighted gene co-expression network and gene set enrichment analyses confirmed downregulation of pathways involved in branching blood vessel morphogenesis but also revealed novel transcriptional mechanisms of action, including control of microtubule transport and ATP synthesis coupled electron transport. DMFu induced a decrease in maximal mitochondrial respiration, an increase in glycolysis and glycolytic capacity and reduced Complex II protein expression of the SDHB subunit on western blot. Such metabolic rewiring may limit the bioenergetic demands required to support angiogenic growth. With therapies available for nAMD being both limited and invasive, DMFu is a contender to be rapidly repurposed as an oral, patient-friendly therapeutic alternative.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


