反式-2-癸烯醛通过诱导ROS积累和线粒体功能障碍抑制辣椒疫霉
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
辣椒疫霉(Phytophthora capsici)是一种极具破坏性的植物致病卵菌,对全球农业生产构成严重威胁。反式-2-癸烯醛是一种天然的植物源性不饱和脂肪醛,其对植物病原体的抗菌性能尚不清楚。本研究系统探讨了其对辣椒疫病毒的抑制作用及其机制。经生物熏蒸,反式-2-癸烯醛对辣椒疫病毒具有较强的抑制活性,EC50值为12.37 μg/mL。它不仅影响正常菌丝形态和超微结构,而且抑制孢子囊的形成、游动孢子的释放和萌发,并呈剂量依赖性。钙白染色显示反式-2-decenal破坏细胞壁完整性。反式-2-decenal处理破坏了细胞膜的完整性和通透性,伴随着DNA和可溶性蛋白的泄漏。此外,反式-2-十烯醛引发细胞内ROS积累,降低谷胱甘肽水平,破坏线粒体膜电位,最终导致细胞功能障碍和死亡。植物试验验证了其对辣椒粉侵染的控制效果。转录组学分析进一步鉴定出2557个差异表达基因,在氮代谢、酪氨酸代谢和苯丙氨酸代谢等关键代谢途径中显著下调。综上所述,这些结果阐明了反式-2-癸烯醛对辣椒疫病的多靶点抑制机制,并突出了其作为一种生态友好剂的潜力,可用于植物病原菌诱导的植物病害的可持续管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
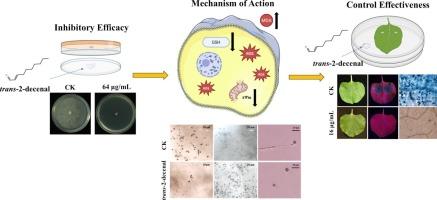
Trans-2-decenal inhibits Phytophthora capsici by inducing ROS accumulation and mitochondrial dysfunction
Phytophthora capsici is a highly destructive plant-pathogenic oomycete that poses a severe threat to global agricultural production. Trans-2-decenal is a natural plant-derived unsaturated aliphatic aldehyde, whose antimicrobial properties against plant pathogens remain poorly understood. In this study, its inhibitory efficacy and underlying mechanisms against P. capsici were systematically investigated. Trans-2-decenal exhibited potent inhibitory activity against P. capsici via biofumigation, with an EC50 value of 12.37 μg/mL. It not only impacted normal hyphal morphology and ultrastructure, but also inhibited sporangia formation, zoospore release, and germination in a dose-dependent manner. Calcofluor white staining showed that trans-2-decenal disrupted cell wall integrity. Trans-2-decenal treatment compromised cell membrane integrity and permeability, accompanied by DNA and soluble protein leakage. Additionally, trans-2-decenal triggered intracellular ROS accumulation, reduced glutathione levels, and disrupted mitochondrial membrane potential, ultimately leading to cellular dysfunction and death. In planta assays validated its robust control efficacy against P. capsici infection. Transcriptomic analysis further identified 2557 differentially expressed genes, with significant downregulation in critical metabolic pathways such as nitrogen metabolism, tyrosine metabolism and phenylalanine metabolism. Taken together, these results elucidate a multitarget inhibitory mechanism of trans-2-decenal against P. capsici, and highlight its potential as an eco-friendly agent for the sustainable management of Phytophthora-induced plant diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


