BbCFEM7通过与蒙氏肠球菌竞争铁元素来增强昆虫病原真菌的毒力
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
真菌胞外膜结构域蛋白(CFEM)是真菌中一类独特的胞外膜蛋白。球孢白僵菌是一种昆虫病原真菌,在农林害虫的生物防治中起着至关重要的作用。然而,昆虫病原真菌与宿主肠道微生物相互作用的机制鲜有报道。评价了BbCFEM7基因在真菌生长、毒力和免疫防御中的作用。根据肠道微生物多样性结果,BbCFEM7的缺失对昆虫肠道细菌群落,尤其是肠球菌有相当大的影响。与野生型菌株(WT)相比,ΔBbCFEM7菌株感染显著影响了昆虫肠道和血淋巴中脂肪酸(包括短链脂肪酸)、有机酸和吲哚代谢物的含量。从真菌感染的昆虫血淋巴中分离出蒙地肠球菌。体外和体内实验表明,真菌和细菌通过快速获取铁对彼此的生长产生抑制作用。我们的研究表明,当球孢单胞菌感染昆虫时,BbCFEM7与从肠道逃逸到血淋巴的蒙氏单胞菌竞争铁离子,导致肠道和血淋巴代谢失调,从而逃避宿主免疫,增强真菌毒力。本研究结果丰富了真菌CFEM结构域蛋白的宿主感染机制,为补充安娜卡列尼娜原理(Anna Karenina Principle, AKP),进一步揭示昆虫肠道细菌与病原真菌的相互作用提供了新的参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
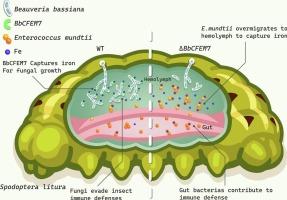
BbCFEM7 contributes to the virulence of entomopathogenic fungi by competing for iron with Enterococcus mundtii
Common in fungal extracellular membrane (CFEM) domain proteins are a unique family of extracellular membrane proteins in fungi. Beauveria bassiana, as a entomopathogenic fungus, plays a critical role in the biological control of agricultural and forestry pests. However, the mechanism of interaction between entomopathogenic fungi and host gut microbes is rarely reported. The contribution of the BbCFEM7 gene to fungal growth virulence and immune defense was evaluated. According to the gut microbial diversity results, the absence of BbCFEM7 had a considerable influence on the insect gut bacterial communities, especially enterococcus. The infection with ΔBbCFEM7 strains significantly affected the contents of fatty acids (including short-chain fatty acids), organic acids, and indole metabolites in insect gut and hemolymph when compared to wild type strain (WT). Enterococcus mundtii was isolated in a fungus-infected insect hemolymph. According to in vitro and in vivo experiments, fungi and bacteria exerted an inhibitory effect on the growth of each other through rapid iron acquisition. Our study demonstrated that when B. bassiana infected insects, BbCFEM7 competed for iron ions with the E. mundtii escaping from the gut to the hemolymph, inducing metabolic dysregulation in the gut and hemolymph, thereby evading host immunity and augmenting fungal virulence. Enriching the host infection mechanism of fungal CFEM domain proteins, the results of this study provide a new reference for supplementing the Anna Karenina Principle (AKP) and further revealing the interaction between insect gut bacteria and pathogenic fungi.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


