合成气合成高级醇:参数空间探索及概念工艺设计
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
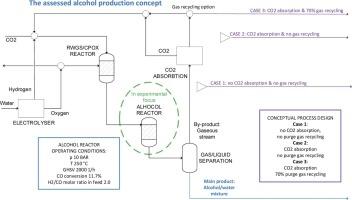
考察了在11% cufecok /SiO2催化剂上合成高级醇的不同反应条件。测试温度范围为250 ~ 300℃,压力为10 ~ 30 bar,气体每小时空速为1000 ~ 3000 mL h-1gcat-1,均在H2:CO比为2的恒定条件下进行。在低CO转化率下,有可能实现对醇的高选择性,而在高转化率下,形成CO2和烷烃的副反应更占主导地位。在实验工作的基础上,考虑主要产物、水相酒精混合物和气态副产物,进行了酒精生产的概念工艺设计。气态副产物的组成H2:CO的摩尔比为2,适合下游的费托合成和/或甲醇合成,其他化合物含量低。由于只有在低CO转化率下才有高醇选择性,因此在概念工艺设计中考虑了气体的回收,发现通过回收98%的吸收CO2和70%的气态副产物,可以实现50%的电力需求和20%的反应器体积减少。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis of higher alcohols from syngas: exploring the parameter space and conceptual process design
Different reaction conditions were evaluated in the synthesis of higher alcohols over an 11%-CuFeCoK/SiO2 catalyst. The temperature range tested was 250 to 300 °C, pressure 10 to 30 bar, and the gas hourly space velocity of 1000 to 3000 mL h-1gcat-1 all under a constant H2:CO ratio of 2. At low CO conversions it was possible to achieve high selectivity to alcohols, while at high conversions side reactions forming CO2 and alkanes became more dominating. Based on the experimental work, conceptual process design for alcohol production was conducted considering the main product, the aqueous alcohol mixture, and a gaseous by-product. The composition of the gaseous by-product had a H2:CO molar ratio of 2, making it suitable for downstream Fischer-Tropsch and/or methanol synthesis, and a low content of other compounds. Due to high alcohol selectivity only at low CO conversion, recycling of the gases was taken into account in the conceptual process design and it was found that by recycling 98% of the absorbed CO2 and 70% of the gaseous by-products a 50% electricity demand and 20% reactor volume decrease could be achieved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Research & Design
工程技术-工程:化工
CiteScore
6.10
自引率
7.70%
发文量
623
审稿时长
42 days
期刊介绍:
ChERD aims to be the principal international journal for publication of high quality, original papers in chemical engineering.
Papers showing how research results can be used in chemical engineering design, and accounts of experimental or theoretical research work bringing new perspectives to established principles, highlighting unsolved problems or indicating directions for future research, are particularly welcome. Contributions that deal with new developments in plant or processes and that can be given quantitative expression are encouraged. The journal is especially interested in papers that extend the boundaries of traditional chemical engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


