Cu/Fe3O4催化剂上水气转换反应的还原活化Cu+/Cu0协同优化
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
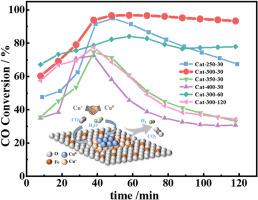
Cu/Fe3O4催化剂具有优异的水气转换(WGS)反应活性,可替代有毒的Fe-Cr HTS催化剂。在本研究中,采用还原诱导活化策略合成Cu/Fe3O4催化剂,控制Cu+和Cu0的生成。系统研究表明,将还原温度从250℃提高到400℃,不仅提高了Cu+/(Cu0+Cu+)比,而且大大减小了Cu纳米颗粒的尺寸,从而协同增强了CO和H2O的吸附活化动力学。优化后的Cu/Fe3O4催化剂在300°C还原后,表现出优异的WGS反应活性,在340°C时CO转化率为93.9%,优于已有报道的商用Fe-Cr催化剂。这种优异的性能可归因于Cu+/(Cu0+Cu+)比(45.0%)平衡良好,以及以超小Cu (9.29 nm)和Cu2O (6.16 nm)纳米颗粒为特征的精细纳米结构。明确了CO和H2O的最佳吸附活化位点分别在Cu+和Cu0位点。Cu+-Cu0位点的协同作用显著加快了WGS反应的进程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergistic Cu+/Cu0 optimization via reduction-induced activation for water-gas shift reaction over Cu/Fe3O4 catalyst
Cu/Fe3O4 catalysts have excellent water-gas shift (WGS) reaction activity, which served as a replacement for toxic Fe-Cr HTS catalysts. In this study, a reduction-induced activation strategy was applied to synthesize Cu/Fe3O4 catalysts with controlled generation of Cu+ and Cu0 species. Systematic investigations demonstrated that increasing the reduction temperature from 250 to 400 °C not only raised the Cu+/(Cu0+Cu+) ratio but also reduced Cu nanoparticle size highly, thereby synergistically enhancing CO and H2O adsorption-activation kinetics. The optimized Cu/Fe3O4 catalyst, reduced at 300 °C, exhibited outstanding WGS reaction activity, gaining a CO conversion of 93.9 % at 340 °C, which outperformed the reported commercial Fe-Cr catalysts. This superior performance can be attributed to a well-balanced Cu+/(Cu0+Cu+) ratio (45.0 %) and a refined nanostructure, characterized by ultrasmall Cu (9.29 nm) and Cu2O (6.16 nm) nanoparticles. It was clarified that the optimal adsorption activation sites for CO and H2O were at the Cu+ and Cu0 sites, respectively. What's more, synergistic effect of Cu+-Cu0 sites significantly accelerated the process of WGS reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


