具有抗单纯疱疹病毒活性的高氧吡虫胺类甾体
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
先前未被描述的甾类化合物vernomigeodiins A-D(1-4),与已知的甾醇5-10和三肽醋酸金酰胺(11)一起从非洲药用植物Vernoniastrum migeodii中分离出来(11)。分离的类固醇具有独特的偶联Δ7,9(11)-二烯段和高度氧化侧链的污名烷骨架,偶尔形成双环或三环体系。甾醇1-3、5-9是糖基化的,而4和10是糖基化的。化合物的结构通过一维和二维核磁共振波谱和HRESIMS分析得到。分离得到的甾醇对单纯疱疹病毒2型的抗病毒活性进行了评价。首先,使用标准MTT法评估其在Vero细胞中的细胞毒性,然后使用直接定量PCR评估其抗病毒活性。蚓尾草苷A(1)、B(4)和蚓尾草苷B1(6)在10 μM浓度下均无细胞毒性,但能降低HSV-2病毒DNA水平,IC50值在0.25 ~ 0.56 μM之间。此外,vernoamyoside B(9)具有直接杀病毒作用(IC50 = 1.23±0.15 μM)。利用分子对接的方法研究了抗病毒化合物与特定病毒靶点之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
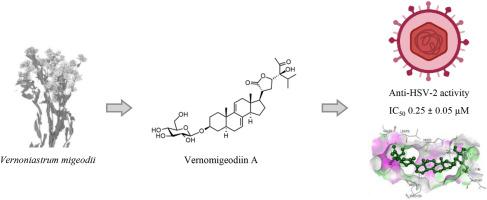
Vernomigeodiins A–D, highly oxygenated stigmastane-type steroids isolated from Vernoniastrum migeodii with anti-herpes simplex virus activity
Previously undescribed steroids vernomigeodiins A–D (1–4), were isolated from the African medicinal plant Vernoniastrum migeodii along with known sterols 5–10 and the tripeptide aurantiamide acetate (11). The isolated steroids featured a stigmastane skeleton with a unique conjugated Δ7,9(11)-diene segment and a highly oxidized side chain, occasionally forming a bi- or tricyclic ring system. Sterols 1–3, 5–9 are glucosylated, whereas 4 and 10 are aglycons. The structures of the compounds were elucidated on the basis of 1D and 2D NMR spectroscopy and HRESIMS analyses. The isolated sterols were evaluated for their antiviral activities against Herpes simplex virus type 2. First, their cytotoxicity was assessed in Vero cells using the standard MTT assay, and direct quantitative PCR was then used to evaluate the antiviral activity. Vernomigeodiins A (1) and B (4) and vernonioside B1 (6) were not cytotoxic up to a concentration of 10 μM but reduced the HSV-2 viral DNA levels with IC50 values between 0.25 and 0.56 μM. In addition, vernoamyoside B (9) showed a direct virucidal effect (IC50 = 1.23 ± 0.15 μM). The interactions between the antiviral compounds and specific viral targets were investigated using molecular docking.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


